Varicella, commonly known as chickenpox, is caused by human herpesvirus type 3, or varicella zoster virus (VZV), one of the nine DNA viruses of the herpesviridae family that may cause disease in humans. Primary infection typically occurs in childhood, although adults may be infected as well, followed by dormancy of the virus in the neurosensory ganglia. The virus may reactivate years to decades later, resulting in herpes zoster (HZ), or shingles.
Here, I discuss the epidemiology, clinical manifestations, complications, diagnosis and evaluation, management and treatment, and prevention of HZ.
Epidemiology
There are approximately 1.2 million new cases of HZ annually in the United States.1 One in three individuals will develop shingles in their lifetime.2 By age 85, more than 50% of unvaccinated individuals will be affected (see “Prevention,” p.41).
The rate of HZ has increased in the last 7 decades. Between 1945 and 1949, the incidence of HZ in the US population was 0.76 cases per 1,000 person-years. Between 2000 and 2007, the rate rose to 3.15 cases per 1,000.3 A recent study on an unvaccinated, immunocompetent population found an incidence rate of 9.92/1,000 person-years, with a recurrence rate of 10.96/1,000 person-years.4 The reasons for this increased rate are unclear.
While HZ is most common in adults between the ages of 60 and 69, children may also develop shingles. Although immunosenescence or immunosuppression may play a role in the reactivation of HZ, greater than 90% of affected patients are immunocompetent.5

Ocular involvement, known as herpes zoster ophthalmicus (HZO), represents 10% to 20% of cases of HZ.6 There are 120,000 to 240,000 cases of HZO annually in the US. The incidence of HZO has risen between 1994 and 2018, with an increase of 3.6% per year in the US. Since 2008, the incidence has decreased in individuals younger than age 21 and older than age 60, while it has increased at a lower rate in middle-aged individuals. Female and Caucasian patients are at highest risk.7
Clinical Manifestations
Varicella is a highly contagious, airborne disease characterized by an itchy vesicular rash, with constitutional symptoms, including fever, headache, and fatigue. Symptoms last 5 to 7 days, and the rash eventually scabs over. Serious complications are rare and include pneumonia and encephalitis.8
Varicella vaccination, introduced in 1995, has decreased the overall number of cases and complications from varicella. Routine immunization is recommended for children in many countries, and, if administered within the first 3 days of infection, decreases the severity of the disease.9 Although it is possible to become reinfected, individuals ordinarily get chickenpox once in their lifetime.
Patients with HZ commonly have a prodromal flu-like syndrome with fatigue, malaise, headache, and low-grade fever for up to a week. Typically, patients experience symptoms of burning, itching, hyperesthesias, or paresthesias in a dermatomal distribution on one side of the body or face for 2 to 4 days. An erythematous maculopapular rash then appears in one or two adjacent dermatomes (localized zoster) and progresses to clustered vesicles. The pain may range from mild to severe. In patients with weak immune systems, the rash may extend to three or more dermatomes and become disseminated zoster. The dermatitis usually crusts over and resolves in 2 to 4 weeks. Permanent scarring or skin pigmentary changes may result. A small percentage of patients who have HZ can have pain in a classic dermatomal distribution without the characteristic rash. This is known as zoster sine herpete.10,11
If the viral infection involves the eye (HZO), vision loss may result. The frontal branch of the trigeminal nerve is most commonly involved in HZO. Involvement of the nasociliary branch may produce vesicles on the root, tip, or side of the nose, termed Hutchinson’s sign, signaling possible ocular involvement.12 Ocular involvement may occur in up to 50%-75% of HZO cases;13 up to 25%-30% of cases will develop chronic or recurrent disease even with appropriate treatment.14
Ocular symptoms include tearing, redness, decreased vision, and irritation. Ophthalmic manifestations may involve every structure of the eye and surrounding structures.14,15
- Lid involvement. In addition to the rash, this may include lid hyperemia and edema, and preseptal cellulitis. Ptosis may result from edema. Scarring is not uncommon after the HZ rash. The lid may lose mobility, and lagophthalmos may result due to cicatricial skin changes and orbicularis oculi paralysis. Resulting exposure keratopathy may ensue.
- Conjunctival inflammation with mucopurulent discharge. This can occur in up to 57% of patients. Conjunctivitis may be membranous, pseudomembranous or follicular. Vesicles may be present on the bulbar or palpebral conjunctiva, which may rupture and cause inflammation that may lead to cicatricial changes, such as symblepharon, in severe cases.
- Episcleritis and scleritis. This may be localized or diffuse. Scleral atrophy may occur in later stages.
- Corneal manifestations. Approximately 65% of patients may have corneal involvement, including early findings of punctate keratopathy and pseudodendrites. Later, subepithelial and interstitial infiltrates, disciform keratitis, endotheliitis, and late mucous plaques may occur. Neurotrophic keratopathy may lead to ocular surface dryness, persistent epithelial defects, ulceration, and possible perforation. Lipid keratopathy and scarring may also lead to serious visual loss.
- Anterior uveitis. This may be recurrent and associated with sectoral iris atrophy and synechiae.
- Posterior segment inflammation. Necrotizing retinitis (acute retinal necrosis, progressive outer retinal necrosis), retinal perivasculitis, retinal detachment, and optic neuritis may occur, but are rare.
- Glaucoma and cataract. These conditions may result as late sequelae of inflammation or long-term topical steroid therapy.
- Diplopia or even complete inability to move the globe. These issues may result from cranial nerve palsies and are often self-limited.
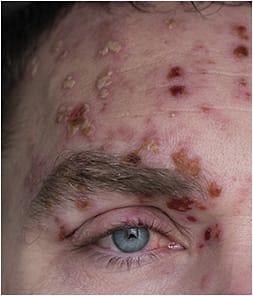
Complications
Postherpetic neuralgia (PHN) is the most common complication of HZ and is caused by damage to affected nerves due to inflammation from viral replication. It is characterized by persistent pain, hyperalgesia, and allodynia beyond 3 months after the onset of shingles.16
PHN occurs in 10% to 18% of patients who have HZ and in 30% of patients who have HZO. It is uncommon in patients younger than age 40. Older patients have greater severity and duration of pain. Other risk factors include severity of prodrome, acute pain, or rash, ocular involvement, diabetes, and immunosuppression. The pain from PHN may be debilitating and, thus, greatly affect quality of life.6,17
Other complications of HZ include bacterial superinfections of the involved skin, most commonly due to streptococcus or staphylococcus species, peripheral nerve palsies, and meningoencephalitis, pneumonitis, and hepatitis. Deafness, paraparesis, and motor neuropathies may also occur. HZ and HZO patients are also at increased risk for stroke,18 acute cardiovascular events,18 severe depression and cancer, particularly lymphoma.19 VZV has also been implicated as a trigger for giant cell arteritis.20
Diagnosis and Evaluation
Diagnostic workup includes a complete medical history, including primary infection history and vaccination. The history should include a review of systems for risk factors, including immunosuppression. The diagnosis of HZ is usually based on clinical presentation.
Testing may be helpful in cases of recurrent dermatitis suspicious for herpes simplex virus, in cases of zoster sine herpete, atypical presentations, or in disseminated zoster. Tzanck smear or Wright stain may be used to test lesions for herpes virus, but these are not specific for VZV. Viral cultures, direct immunofluorescence assay and polymerase chain reaction evaluation of the lesions may also be used to confirm the diagnosis.
Management and Treatment
Systemic antiviral therapy includes the oral guanosine analogues acyclovir (Zovirax, GlaxoSmithKline), valacyclovir (Valtrex, GlaxoSmithKline), and famciclovir (Famvir, Novartis). All three are FDA approved for active HZ and reduce duration of pain and new lesion formation, as well as duration of viral shedding. They also accelerate healing of cutaneous lesions. They do not reduce the incidence of PHN. Acyclovir is the only antiviral agent approved for children with HZ. Intravenous acyclovir is administered to patients who have severe disease or who are immunocompromised. Treatment with antiviral agents should ideally be instituted within 72 hours of rash onset, but may still be of benefit after this initial window if new skin lesions are forming, or ophthalmic or neurologic complications are present. Duration of antiviral therapy is 7-10 days.16,17
Systemic corticosteroids are used adjunctively for pain control, but only concomitantly with oral antiviral therapy. Oral non-steroidal anti-inflammatory agents, acetaminophen, or narcotic medications, such as oxycodone, may be used additionally for pain control, depending on the severity of the pain.
Regarding PHN treatment, commonly used topical agents include lidocaine patches and capsaicin cream or patches. Oral anticonvulsive agents approved for PHN include gabapentin (Neurontin, Pfizer) and pregabalin (Lyrica, Pfizer). Tricyclic antidepressants, including amitryptiline (generic only), nortryptiline (Pamelor, Mallinckrodt Pharmaceuticals) and desipramine (Norpramin, US Pharm Holdings) have all been shown to be effective, but have significant side effects, such as cardiotoxicity. Opioids may be utilized for severe pain as a third line therapy, but the benefit of these agents has not been proven. The opioid tramadol (various manufacturers) has been shown to be effective. Nerve blocks may be utilized in severe cases.16,17
Treatment of HZO is complicated and, in addition to oral antiviral medication, may initially include topical steroids and prophylactic antibiotic drops for ocular surface inflammation, and lubricant drops and gels for dryness or exposure keratopathy. Punctal plugs, scleral lenses, amniotic membrane transplants, autologous serum drops, and cenegermin, a recombinant nerve growth factor (Oxervate, Dompé) may improve ocular surface integrity in cases of neurotrophic keratopathy. Recurrent pseudodendrites can be treated with topical ganciclovir (Zirgan, Laboratoires Théa Corporation). Recurrent or chronic corneal and intraocular inflammation is often treated with topical steroids, which may need to be tapered slowly over months to years. Long-term suppressive oral anti-viral therapy may reduce recurrent inflammation. (Clinical trials are underway). Chronic steroid therapy may lead to increased intraocular pressure and cataracts, necessitating glaucoma therapy and cataract surgery. Other surgical treatments may include partial or complete tarsorrhaphy for ocular surface protection, repair of lid abnormalities, conjunctival flap for non-healing ulcers, and cyanoacrylate glue or tissue adhesive for corneal perforations. Treatment of corneal scarring may include phototherapeutic keratectomy for superficial scarring, lamellar or penetrating keratoplasty, or keratoprosthesis (as a last resort).15-17
Prevention
The live attenuated VZV vaccine (Zostavax, Merck) was initially recommended by the US Advisory Council on Immunization Practices (ACIP)/Centers for Disease Control and Prevention in 2008 for all adults age 60 and older. In 2011, the ACIP expanded its recommendation for use in all age 50 and older, after it was shown to decrease the incidence of HZ 68% in persons age 50-59. Zostavax is administered as a single intramuscular dose. Contraindications include immunosuppression or compromised immune system from HIV/AIDS or other disease, history of cancer affecting the bone marrow or lymphatic system, or active, untreated tuberculosis. The efficacy of Zostavax declines with increasing age.21,22
The adjuvanted non-live recombinant vaccine (Shingrix, GlaxoSmithKline), was approved by the FDA in October 2017 and is over 90% effective at preventing HZ and PHN. It is administered in two intramuscular doses over 2-6 months.
Currently, the ACIP recommends Shingrix for all adults older than age 50, due to its safety and efficacy. This includes people who have had HZ, who have had Zostavax at least 8 weeks prior, who have chronic health conditions, such as diabetes mellitus or systemic lupus erythematosus, who are receiving other vaccines (such as influenza and pneumovax 23 [Merck]) at the same visit, and who are taking low-dose immunosuppressive therapy. The ACIP does not recommend the vaccine for patients who are immunocompromised, but it is not a contraindication, and recommendations will be updated as these populations are studied. The vaccine is contraindicated in those with a history of severe allergy to a component, or after a dose of Shingrix. It should be delayed in those who are pregnant, lactating, or have active shingles.21
In patients with a history of HZO, several cases of reactivated keratitis or iritis23,24 have been reported after Zostavax and Shingrix vaccination in patients who were previously stable. In two patients with chronic disease, but without a history of HZO, acute retinal necrosis developed after Zostavax vaccination.25 Thus, in patients with active HZO, delay in vaccination is likely prudent. Informed consent and careful follow up for at least 6 months after vaccination is recommended in these patients. CP
| AGENT | DOSAGE (ADULT) | ADVERSE EFFECTS | NOTES | COST* |
|---|---|---|---|---|
| ANTIVIRALS | ||||
| Acyclovir | 800 mg orally five times per day for seven days | Diarrhea, encephalopathy, erythema multiforme, headache, malaise, nausea, Stevens- Johnson syndrome, vomiting | Dosing adjustments required for immunocompromised patients (10 mg per kg intravenously every eight hours) and for patients with creatinine clearance ≤ 50 mL per minute per 1.73 m2 (0.83 mL per second per m2) Approved for use in children (10 mg per kg intravenously |
$20 for 45 800-mg generic tablets |
| Famciclovir | 500 mg orally three times per day for seven days | Confusion, headache, nausea, Stevens-Johnson syndrome | Dosing adjustment required for patients with creatinine clearance ≤ 60 mL per minute per 1.73 m2 (1.00 mL per second per m2) | $32 for 21 500-mg generic tablets ($522 for brand name) |
| Valacyclovir (Valtrex) | 1,000 mg orally three times per day for seven days | Similar to acyclovir | Dosing adjustment required for patients with creatinine clearance ≤ 50 mL per minute per 1.73 m2 | $24 for 21 1,000-mg generic tablets ($424 for brand name) |
| ADJUNCTIVE THERAPY | ||||
| Corticosteroids (e.g., prednisone, prednisolone) | Prednisolone: 40 mg orally per day (days 1 to 6), 30 mg per day (days 7 to 10), 20 mg per day (days 11 to 14), 10 mg per day (days 15 to 18), 5 mg per day (days 19 to 21) Prednisone: 60 mg orally per day (days 1 to 7), 30 mg per day (days 8 to 14), 15 mg per day (days 15 to 21) |
Dyspepsia, nausea, vomiting | Associated with accelerated time to crusting and healing of lesions and resolution of pain; no benefit in preventing postherpetic neuralgia | Varies |
| ANTIVIRALS | ||||
| Acetaminophen | 325 to 1,000 mg orally every four to six hours as needed (maximum: 4,000 mg per day) | Headache, hepatotoxicity, hypersensitivity, nausea, rash | Infant and child dosage: 10 to 15 mg per kg orally every four to six hours as needed (maximum: 4,000 mg per day) | $7 for 100 generic tablets |
| Nonsteroidal antiinflammatory drugs (e.g., ibuprofen) | 400 mg orally every four to six hours as needed (maximum: 2,400 mg per day) | Abdominal discomfort, dyspepsia, gastrointestinal bleeding and perforation, myocardial infarction, nausea | Infant and child dosage (sixmonths and older): 5 to 10 mg per kg orally every six to eight hours as needed (maximum: 2,400 mg per day) | $7 for 100 generic tablets |
| *—Estimated retail cost for one treatment course based on information obtained at http://www.goodrx.com and http://www.walgreens.com (accessed April 4, 2017). Information from references 1, and 10 through 13. |
||||
| AGENT | DOSAGE (ADULT) | ADVERSE EFFECTS | NOTES | COST* |
|---|---|---|---|---|
| TOPICAL TREATMENTS | ||||
| Capsaicin 0.075% cream | Four applications per day | Erythema, pain on application, rash | Avoid contact with eyes and mucous membranes; 8% patch available for application by trained clinicians every three months | $18 for 2-oz tube (over the counter) |
| Lidocaine 5% patch | Up to three patches per day | Blisters, local erythema, rash | Avoid in patients with allergy to amide local anesthetics | $219 for 90 generic patches |
| SYSTEMIC TREATMENTS | ||||
| Amitriptyline | Initial dose of 10 to 25 mg orally at bedtime, then increase by 10 to 25 mg per week to target of 75 to 150 mg per day | Blurred vision, constipation, dry mouth, sedation, urinary retention, weight gain | Taper gradually when discontinuing therapy; use caution in older adults; avoid in patients with cardiac arrhythmias, glaucoma, seizure disorder, or suicide risk; avoid concomitant use of tramadol, selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors (risk of serotonin syndrome) | $4 for 30 75-mg generic tablets |
| Gabapentin (Neurontin) | 300 to 600 mg orally three times per day | Dizziness, peripheral edema, sedation, weight gain | Taper dose over seven days when discontinuing therapy; dosing adjustment required for patients with creatinine clearance ≤ 60 mL per minute per 1.73 m2 (1.00 mL per second per m2) | $13 for 90 300-mg generic capsules ($406 for brand name) |
| Pregabalin (Lyrica) | 150 to 300 mg orally per day in two or three divided doses | Dizziness, peripheral edema, sedation, weight gain | Taper dose over seven days when discontinuing therapy; dosing adjustment required for patients with creatinine clearance ≤ 60 mL per minute per 1.73 m2 | $395 for 60 75-mg brand name capsules (generic not available) |
| *—Estimated retail price based on information obtained at http://www.goodrx.com and http://www.walgreens.com (accessed April 4, 2017). Information from references 11, 27, 36, and 37. |
||||
| CLINICAL RECOMMENDATION | EVIDENCE RATING | REFERENCES |
|---|---|---|
| Although herpes zoster typically is diagnosed clinically, if laboratory confirmation is needed, polymerase chain reaction testing of vesicle or other fluids is preferred for diagnosis because of its highsensitivity (95%) and specificity (100%). | C | 7 |
| Acyclovir, valacyclovir (Valtrex), and famciclovir are effective treatments for herpes zoster and ideally should be started within 72 hours of the appearance of the rash to decrease the duration of symptoms and severity of pain. | B | B 1, 2, 7, 14-16 |
| Capsaicin 8% patches, applied for 30 to 90 minutes, provide effective pain relief for patients with postherpetic neuralgia. | A | 40 |
| Gabapentin (Neurontin) and pregabalin (Lyrica) can be used for treatment of postherpetic neuralgia. | A | 42 |
| Amitriptyline, nortriptyline (Pamelor), and desipramine can be used for pain relief in patients with postherpetic neuralgia (number needed to treat = 3; 95% confidence interval, 2 to 4). | A | 26, 44 |
| The varicella zoster virus vaccine (Zostavax) should be given to patients 60 years and older, but it is contraindicated in those who are immunosuppressed, have human immunodeficiency virus infection and CD4 lymphocyte counts less than 200 per mm3 (0.20 × 109 per L), are undergoing cancer treatment, or who have cancer affecting the bones or lymphatic system. | A | 48, 50, 51 |
| A = consistent, good-quality patient-oriented evidence; B = inconsistent or limited-quality patient-oriented evidence; C = consensus, disease-oriented evidence, usual practice, expert opinion, or case series. For information about the SORT evidence rating system, go to http://www.aafp.org/afpsort . | ||
References
- Suaya JA, Chen S-Y, Li Q, Burstin, SJ, Myron JL. Incidence of herpes zoster and persistent post-zoster pain in adults with or without diabetes in the United States. Open Forum Infect Dis. 2014;1(2):ofu049. doi: 10.1093/ofid/ofu049. eCollection 2014 Sep.
- Harpaz R, Ortega-Sanchez IR, Seward JF. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MWR Recomm Rep. 20086;57(RR-5):1-30; quiz CE2-4.
- Kawai K, Yawn BP, Wollan P, Harpaz R. Increasing incidence of herpes zoster over a 60-year period from a population-based study. Clin Infect Dis. 2016;63(2):221-6.
- Tseng HF, Bruxvoort K, Ackerson B, Luo Y, Tanenbaum H, Tian Y, et al. The epidemiology of herpes zoster in immunocompetent, unvaccinated adults ≥50 years old: incidence, complications, hospitalization, mortality, and recurrence J Infect Dis. 2019;jiz652. doi: 10.1093/infdis/jiz652. Online ahead of print.
- Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341-9.
- Ragozzino MW, Melton LJ, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine. 1982;(5):310-316.
- Kong CL, Thompson RR, Porco TC, Kim E, Acharya NR. Incidence rate of herpes zoster ophthalmicus. A retrospective cohort study from 1994 through 2018. Ophthalmology. 2020;127(3):324–330.
- Kennedy P, Gershon AA. Clinical features of varicella-zoster virus infection. Viruses. 2018;10(11):609.
- Routine Varicella Vaccination | For Providers | CDC. https://www.cdc.gov/vaccines/vpd/varicella/hcp/recommendations.html . Accessed June 9, 2020.
- Schmader K. Herpes zoster. Clin Geriatr Med. 2016;32(3):539‐553.
- Lewis GW. Zoster sine herpete. Br Med J. 1958;2:418–421.
- Zaal MJW, Völker-Dieben HJ, D'Amaro J. Prognostic value of Hutchinson's sign in acute herpes zoster ophthalmicus. Graefes Arch Clin Exp Ophthalmol. 2003 Mar;241(3):187-191.
- Liesegang, Thomas J. Diagnosis and therapy of herpes zoster ophthalmicus. Ophthalmology. 1991;98(8):1216-29
- Liesegang T. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008 Feb;115(2 Suppl):S3-12.
- Kaufman SC. Anterior segment complications of herpes zoster ophthalmicus. Ophthalmology. 2008;115:(2 Suppl):S24-32.
- Saguil A, Kane S, Mercado M, Lauters R. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician. 2017;96(10):656-663.
- Cohen EJ. Management and prevention of herpes zoster ocular disease. Cornea. 2015;34 Suppl10:S3‐S8.
- Wu P-H, Chuang Y-S, Lin Y-T. Does herpes zoster increase the risk of stroke and myocardial infarction? A comprehensive review. J Clin Med. 2019 Apr 22;8(4):547.
- Iglar K, Kopp A, Glazier RH. Herpes zoster as a marker of underlying malignancy. Open Med. 2013 Jun 18;7(2):e68-73. eCollection 2013.
- Gilden D, Nagel MA. Varicella zoster virus and giant cell arteritis. Curr Opin Infect Dis. 2016;29(3):275-9.
- Gagliardi AMZ, Andriolo BNG, Torloni MR, Soares BGO, de Oliveira Gomes J, Andriolo RB, Canteiro Cruz E. Vaccines for preventing herpes zoster in older adults. Cochrane Database of Systematic Reviews 2019, Issue 11.
- Vrcek I, Choudhury E, Durairaj V. Herpes Zoster Ophthalmicus: A review for the internist. Am J Med. 2017;130(1):21-26.
- Lehmann A, Matoba A: Reactivation of herpes zoster stromal keratitis after HZ/su adjuvanted herpes zoster subunit vaccine. Ophthalmology. 2018;125(11):1682.
- Jastrzebski A, Brownstein S, Ziai S, Solin S, Kay L, Jackson WB. Reactivation of herpes zoster keratitis with corneal perforation after zoster vaccination. Cornea. 2017;36(6):740–742.
- Charkoudian LD, Kaiser GM, Steinmetz RL, Srivastava SK. Acute retinal necrosis after herpes zoster vaccination. Ophthalmol. 2011 Nov;129(11):1495-1497.