In preparation for cataract surgery, accurate IOL measurements are of paramount importance for good outcomes and a satisfied patient. Irregularities on the ocular surface can influence the accuracy of IOL calculations. Some common “lumps and bumps” on the ocular surface are Salzmann’s nodules, epithelial basement membrane dystrophy (EBMD) and pterygia.
Here, I discuss these conditions and their treatments, so these patients can achieve an uneventful cataract surgery.
Salzmann’s Nodules
Salzmann nodular degeneration can occur anywhere on the cornea, although more often peripherally. It is characterized by greyish-white, elevated nodules evident on slit lamp examination (Figure 1). What precipitates the formation of the nodules is not fully understood.
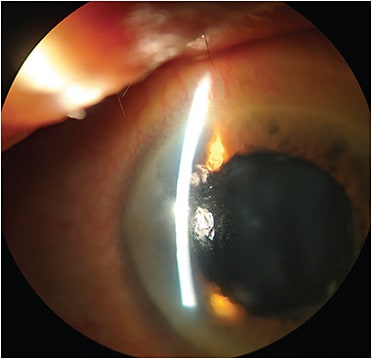
Patients who have Salzmann’s nodules often report foreign body sensation and distorted vision caused by the irregular astigmatism due to the nodules, but can also be asymptomatic. These patients may have a prior history of ocular inflammation, and most have co-existing meibomian gland dysfunction and dry eye disease, which requires management as well.
In the context of cataract surgery, Salzmann’s nodules, even when located in the periphery, can cause central flattening and astigmatism.1 Topography mires appear distorted due to these lesions. On tomography, areas of corneal elevation are correlated to thickening (Figure 2).
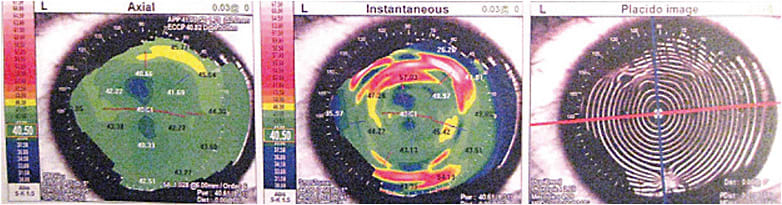
The definitive treatment for Salzmann’s nodules is surgical removal using techniques of superficial keratectomy, with or without adjuvant alcohol, mitomycin C (MMC), PTK and amniotic membrane graft.2
I have found success in performing superficial keratectomy in the minor procedure room, using topical anesthesia with the eye prepped with povidone-iodine, draping to keep the eyelashes covered, and a lid speculum.
A 6400 Beaver Blade (BVI) is used with gentle force on a dry corneal surface in a manner akin to removing the char off burnt toast to avoid inadvertent cutting into the stroma. Once the epithelial layer is removed in an area adjacent to the Salzmann’s nodule, the plane of Bowman’s membrane is evident, and the nodule may be peeled using 0.12 forceps in a motion like a capsulorrhexis. A few drops of balanced salt solution on the corneal surface mimics how the corneal surface will appear once re-epithelialized, so further debridement can be done to any remaining areas of elevation, if need be.
Topical antibiotics are applied, along with a bandage contact lens, which is removed usually 1 week later, or once the corneal surface has re-epithelialized. A tapering course of topical steroids is also instituted postoperatively.
The first 48 hours after superficial keratectomy are typically the most uncomfortable for the patient, so oral analgesics should be prescribed. The patient can be advised that vision usually improves by 1 week, but may take about 4 to 6 weeks to fully stabilize. Applying a Weck-Cel Spear (BVI) soaked in topical MMC 0.02% for no more than 2 minutes, followed by rinsing with balanced salt solution, can be considered for recurrent nodules or for those that are vascularized and located adjacent to the limbus.
Once the corneal epithelium has fully healed and remodelled, at about 6 weeks postoperatively, use of diagnostic imaging is helpful to evaluate the improvements in corneal regularity and astigmatism. Topography and refraction are performed, and can be repeated to ensure stability prior to booking the patient for IOL measurements.
In a study of 42 eyes that underwent superficial keratectomy for Salzmann’s nodules, refractive astigmatism was significantly improved, and no recurrences occurred after a mean follow-up of approximately 4.6 months.3
EBMD
Also known as anterior basement membrane dystrophy (ABMD), Cogan’s microcystic dystrophy, and map-dot-fingerprint dystrophy, EBMD presents as cysts, fingerprint and map-like lines that demonstrate a negative staining pattern with fluorescein instilled under cobalt blue light (Figure 3). Additionally, mires on topographic Placido disc imaging can show corneal irregularity (Figure 4).
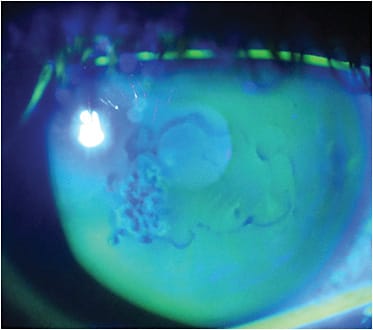
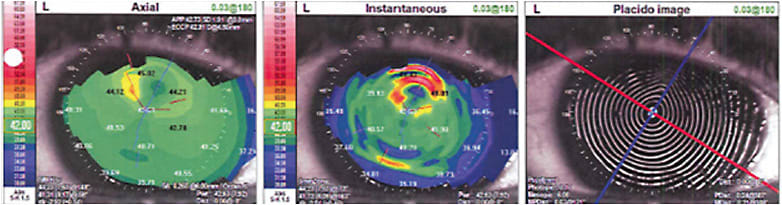
Patients can present with symptoms of decreased or fluctuating vision. Episodes of pain, photophobia, and/or tearing may also occur from EBMD-associated recurrent erosions.
In the context of cataract surgery, visually significant EBMD (typically involving the central visual axis) should be treated prior to IOL calculations.
I have had success with epithelial debridement at the slit lamp or in the minor procedure room, with the patient under topical anesthesia. Additionally, the eye is prepped with povidone-iodine, the lids draped to keep the eyelashes covered, and a lid speculum is inserted. Weck-Cel Spears (BVI), or a 6400 Beaver Mini-Blade (BVI), are used with a gentle sweeping motion on a dry corneal surface, as if spreading butter on toast, to avoid inadvertent cutting into the stroma. All loose epithelium is removed, which often leaves the total Bowman’s membrane bare. The postoperative care is similar to the previously described regimen for Salzmann’s nodule superficial keratectomy. In patients with recurrent erosion symptoms, I will often leave the bandage contact lens in place for an extended 6 weeks with topical antibiotic coverage to ensure that the epithelial layer has had sufficient time to remodel.
Regarding cataract surgery, the improvement in vision achieved after treatment by debridement may actually obviate the need for cataract surgery. It is also important to note that untreated visually significant EBMD may cause the vision after cataract surgery to remain poor, resulting in an unhappy patient. If peripheral areas of EBMD are left untreated prior to cataract surgery, the surgeon should ensure that the ocular surface does not dry out excessively intraoperatively, which can lead to epithelial sloughing. The surgeon can ensure this via frequent re-wetting of the cornea during the case or by coating the epithelium with Cornea Protect (Bausch + Lomb Surgical) or viscoelastic. Should sloughing occur, the loose epithelium can be debrided with a Weck-Cel Spear (BVI) and a bandage contact lens applied.
Patients should be advised they may experience some increased discomfort and pain for the first 48 hours and be provided with a prescription for oral analgesics.
Epithelial debridement alone in patients with recurrent erosion symptoms due to EBMD is often insufficient, requiring adjuvant treatments, such as anterior stromal puncture, PTK, diamond burr dusting of Bowman’s membrane, or alcohol delamination. This is beyond the scope of this discussion.
In 83 eyes that had EBMD, mean best-corrected visual acuity (20/47 to 20/40, P = 0.033), magnitude of refractive astigmatism (1.76 ± 1.83 D to 1.15 ± 1.08 D, P = 0.010), and magnitude of corneal astigmatism (1.44 ± 0.88 D to 1.06 ± 0.88 D, P = 0.022) all significantly improved after epithelial debridement.3
Another study looked at eyes that had cataract and EBMD or Salzmann’s nodules scheduled for superficial keratectomy or PTK that underwent baseline biometry and repeat biometry 30 days or more postoperatively.4 In the 26 eyes that had EBMD, the mean absolute intersession difference showed an increase in mean K values (P < .001) and a change in IOL spherical power, predicting a postoperative spherical equivalent (SE) closest to zero (P < .001) in 21 of 26 eyes (8 = 0.5 diopter [D]; 9 = 1.0 D; 4 >1.0 D). In toric IOL-eligible eyes, the recommended IOL toricity changed for 16 of 24 eyes, with a mean cylinder power change of 1.2 D. In the 13 eyes that had Salzmann’s nodules, the mean absolute intersession difference showed an increase in mean K values (P = .023) and a change in IOL spherical power, predicting a postoperative spherical equivalent closest to zero (P < .001) in 11 of 13 eyes (3 = 0.5 D; 3 = 1.0 D; 5 >1.0 D). The recommended IOL toricity changed for 10 of 11 eyes (mean cylinder power change 1.5 D)4
Pterygium
This common ocular surface lesion is associated with UV sunlight exposure. Diagnosis is based on its classic clinical appearance at the nasal and/or temporal limbus. As the lesion increases in size, it leads to increasing with-the-rule astigmatism, distortion of vision, discomfort and red eye.
Pterygium surgery should be performed prior to IOL calculations and cataract surgery. One study of 30 eyes from 26 patients who had primary and recurrent pterygium and underwent primary pterygium surgery showed that after pterygium surgery, there was a significantly improved surface regularity index (1.96+/-1.08 to 1.09+/-0.76, P<.001); mean surface asymmetry index (3.05+/-2.85 to 1.39+/-1.70, P=.003); and mean topographic astigmatism (4.65+/-3.02 D to 2.33+/-2.26 D, P=.003).5
In 2013, the American Academy of Ophthalmology published “Options and Adjuvants in Surgery for Pteryium.”6 The paper examined the results of 51 randomized control trials that had a follow-up of at least 6 months and sought, in part, to determine which surgical options and adjuvants are safest for treating primary and recurrent pteryium. The conclusions from the report: Excision of pterygium leaving bare sclera had unacceptably high recurrence rates up to 80%; conjunctival or limbal autograft was superior to amniotic membrane graft surgery in reducing recurrence rates; intraoperative MMC and conjunctival or limbal autograft reduced recurrence rates; and intraoperative MMC with conjunctival or limbal autograft decreased recurrence rates when compared to use of MMC or autograft alone.
In 2017, the ASCRS Cornea Clinical Committee published a practical surgical guide titled, “Surgical techniques and adjuvants for the management of primary and recurrent pterygia.”7 In this guide, the surgical steps for pterygium excision and conjunctival autografting are described in detail, including use of fibrin glue adhesive and amniotic membrane grafting. Options to manage simple and complex recurrences are also reviewed.
For primary pterygiums, I have found success in excising the head, ensuring a smooth corneal surface and limbus (avoiding too deep a dissection into the stroma, which can induce irregular astigmatism), performing a generous tenotomy, allowing the conjunctiva to recess, recovering a thin superior conjunctival autograft, leaving a 1 mm rim of limbus intact, and securing the autograft with fibrin glue adhesive.
A Smooth Transition
Irregularities on the ocular surface can disrupt the tear film, cause irregular astigmatism, and affect corneal keratometry. Prior to IOL measurements and cataract surgery, management of such conditions should include confirmation of refractive stability prior to IOL measurements and cataract surgery. Calculation accuracy, especially for an advanced technology IOL, is crucial to achieving a satisfactory outcome after cataract surgery. It is also important to note that most of these patients have co-existing meibomian gland dysfunction and/or dry eye disease, which can be exacerbated after cataract surgery. Following guidelines, such as those detailed in the algorithm published by the ASCRS Corneal Clinical Committee, can aid in treating visually significant ocular surface disease prior to surgery.8 CP
References:
- Koch DD. Impact of Salzmann’s lesions on corneal curvature. J Cataract Refract Surg. 1995;21(2):111-112.
- Paranjpe V, Galor A, Monsalve P, Dubovy SR, Karp CL. Salzmann nodular degeneration: prevalence, impact, and management strategies. Clin Ophthalmol. 2019;13:1305-1314.
- Bae SS, Chan CC. Superficial keratectomy: indications and outcomes. Can J Ophthalmol. 2018;53(6):553-559.
- Goerlitz-Jessen MF, Gupta PK, Kim T. Impact of epithelial basement membrane dystrophy and Salzmann nodular degeneration on biometry measurements. J Cataract Refract Surg. 2019;45(8):1119-1123.
- Yagmur M, Ozcan AA, Sari S, Ersöz TR. Visual acuity and corneal topographic changes related with pterygium surgery. J Refract Surg. 2005;21(2):166-170.
- Kaufman SC, Jacobs DS, Lee WB, Deng SX, Rosenblatt MI, Shtein RM. Options and adjuvants in surgery for pterygium: a report by the American Academy of Ophthalmology. Ophthalmology. 2013;120(1):201-208.
- Hovanesian JA, Starr CE, Vroman DT, et al. Surgical techniques and adjuvants for the management of primary and recurrent pterygia. J Cataract Refract Surg. 2017;43(3):405-419.
- Starr CE, Gupta PK, Farid M, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45(5):669-684.









