SARS-CoV-2 Seen in Swabs of Conjunctiva and Tears
A small preponderance of SARS-CoV-2 (COVID-19) was noted in the conjunctival swabs and tears of 19 post-mortem corneas, reports a recent study in The Ocular Surface.1
Specifically, out of 132 ocular tissues of 33 donors, 13% were positive for the virus, which has taken the lives of over 500,000 Americans, at press time. Additionally, of the virus-positive donors, 6 had COVID-19-positive post-mortem nasopharyngeal swabs, and 8 showed positive post-mortem anti-SARS-CoV-2 IgG levels. Further, 3 conjunctival, 1 anterior corneal, 5 posterior corneal and 3 vitreous swabs were positive for SARS-CoV-2 RNA, among 20 eyes taken from 10 donors who had COVID-19. Finally, a SARS-CoV-2 spike and envelope proteins were identified in the corneas’ epithelial layer appropriated sans povidone-iodine (PVP–I) disinfection.
As a result of these findings, the study’s researchers say it is critical that post-mortem nasopharyngeal polymerase chain reaction testing and a PVP-I disinfection protocol be employed to eradicate any tissue containing SARS-CoV-2 being used for corneal transplantation.
Reference:
- Ocular Surf. 2021 Jan;19:322-329.
Alcohol-Based Sanitizer Causing Toxic Keratopathy in Children
Children are experiencing toxic keratopathy from unintentional contact between alcohol-based sanitizer and the eye, according to a recent JAMA Ophthalmology case series.1

The author notes four steps can be taken to prevent this: (1) Promoting hand washing with soap and water vs. sanitizer, especially at home; (2) teaching and training children how to use hand sanitizers; (3) having separate dispensers at shops and malls for children, preferably at a lower height (i.e., below face level); and (4) placing caution signs next to sanitizer dispensers.
Reference:
- JAMA Ophthalmol. Published online January 21, 2021.
UV-Emitting Germicidal Lamp Use Linked With Photokeratitis
Patients presented with acute ocular surface pain, post UV-emitting germicidal lamp exposure, that was diagnosed as photokeratitis, reports a recent case series in Ocular Immunology and Inflammation.
Specifically, visual acuity was 20/30 or better, and anterior segment exam showed differing degrees of conjunctival injection and diffusely distributed punctate epithelial erosions.
The study’s authors say patients should “follow manufacturer recommendations when using UV-emitting germicidal lamps, and avoid direct exposure to the ocular surface.”
Reference:
- Ocul Immunol Inflamm. 2020 Nov 20;1-5.
High Body Mass Index Linked With Keratoconus in Adolescents
Obese and overweight young adults appear more likely to develop keratoconus (KC) vs. their normal weight peers, reports a recent study in the American Journal of Ophthalmology.
Specifically, obese young adults had a greater prevalence (270/100,000) of KC vs. overweight (179/100,000), typical weight (154/100,000), and underweight (141/100,000) young adults. Additionally, the odds ratio for obese adolescents was 1.50 (95% confidence interval [CI] 1.22-1.83; P < .05) compared with the typical weight group, the odds ratio for overweight young adults was 1.42 (95% CI 1.08-1.92; P < .05), and the odds ratio for underweight young adults was 0.84 (95% CI 0.65-1.09; P = .18). This was after adjusting for the confounding factors of age, country of origin, gender, height and socioeconomic status.
The nationwide Israeli, population-based study was comprised of 579,946 males and females between age 16 and 19.9 who attended the draft board as military service candidates between 2006 and 2014, and underwent an eye exam. The participants were divided among four groups based on their adjusted body mass index (BMI) percentiles.
Given the study’s findings, the study’s researchers say, “BMI should be considered a risk factor for keratoconus, and further research should elucidate how obesity is involved in the progress of keratoconus.”
Reference:
- Am J Ophthalmol. 2020 Dec 9;224:200-206.
Eyebank Pioneer Passes
Frederick N. Griffith, creator of the Medical Eye Bank of Maryland and, subsequently, Tissue Banks International, which has 26 subsidiaries nationwide, has passed away at age 88.
“Frederick was a great visionary and an incredible human being,” says Mahmood Farazdaghi, past president of the International Federation of Eye Banks. “... He organized and revolutionized recovery, distribution and utilization of donated corneas in the United States and then introduced, developed and organized modern eye banking globally.

Mr. Farazdaghi points out the creation of the central national distribution for donated corneas as one of Mr. Griffith’s many contributions to the field.
“Corneas were shipped for scheduled surgery from the eye bank of one state to a hospital of another, so no cornea was expired or wasted, and the patient waiting list for cornea transplantation was eliminated,” he explains.
Mr. Griffith’s efforts have reached South Africa, Sudan, Egypt, Ethiopia, Syria, Lebanon, Israel, Greece, Turkey, Germany, Poland, Czech, Slovak, Bulgaria, Croatia, UK, India, Nepal, Bangladesh, China, Union of Myanmar, Philippines, Singapore and Australia.
“All his tremendous achievements and contributions would not have been possible without the unwavering support and passionate involvement of his wife, partner and travel companion, Ms. Beatrice Griffith,” Mr. Farazdaghi adds.
Mr. Griffith is survived by his wife of 61 years, two sons, a daughter and four grandchildren, reports The Baltimore Sun.
Antibody-Based Dry Eye Disease Treatment Under Development
A broad-spectrum immunomodulatory eye drop for severe dry eye disease (DED) and ocular surface disease, resulting from inflammatory and immune system disorders, is being created by University of Illinois at Chicago (UIC) researchers for severe DED and ocular surface disease, resulting from inflammatory and immune system disorders, according to a UIC press release. The researchers have been given a 5-year, $10.15 million grant for the treatment, which will fund preclinical data that backs a commercial application to the FDA to allow for phased clinical trials.
The treatment will contain pooled human immune globulins as an active ingredient. This is because they have broad-spectrum immunomodulatory actions using anti-idiotypic, molecular and cellular mechanisms.
Sandeep Jain, MD, a UIC College of Medicine professor of Ophthalmology, and principal investigator, received the grant from the NEI for a “Translational Research Program to Develop Novel Therapies and Devices for the Treatment of Visual System Disorders.”
Dr. Jain has experience in discovering the presence of neutrophil extracellular traps on the ocular surface of DED patients, as well as anti-citrullinated proteins and autoantibodies in the tears.
Student and Researcher Creates Video to improve Corneal Infection Diagnosis
Pauline Khoo, a PhD candidate at the University of Sydney, and a researcher at the University’s Save Sight Institute (www.sydney.edu.au/save-sight-institute/ ), has created a video to improve both the identification and treatment of microbial keratitis, according to a Save Sight Institute-issued press release.
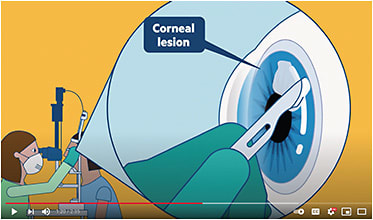
Specifically, the video details the best practices for acquiring an infected tissue sample via a corneal scrape. (See: www.youtube.com/watch?v=rNdE4B5zU6s to watch the 2:35 video.)
“The faster the organism causing the infection is identified, the faster a patient can receive the right treatment, reducing the likelihood of complications,” Ms. Khoo told the Save Sight Institute.
Her focus of study for her PhD is dry eye disease, specifically, improving patient outcomes via a web-based tool that enables doctors to analyze, track and report on patient outcomes from various treatments and compare them to discern what treatment is most effective.
Meibomian Gland Findings Common in Patients Presenting for Refractive Surgery Evaluations
Meibomian gland atrophy and tortuosity are a frequent finding in patients who come in for refractive surgery evaluations, according to a recent study in Clinical Ophthalmology.1
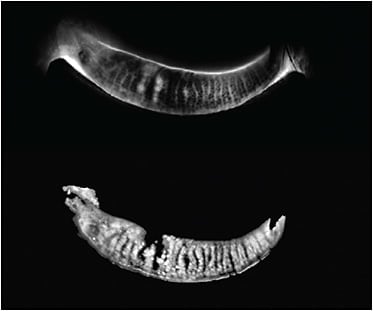
Specifically, roughly 73% of patients had evidence of meibomian gland atrophy, and roughly 70% had indications of tortuosity, with most patients in the atrophy group having the mild form. The study was comprised of 120 patients (49 males) ages 21 to 62.
As a result, of these findings, the study’s researchers say clinicians should consider employing meibography into the refractive surgery evaluation, and, if meibomian gland issues are discovered, proactively treat them, as the condition is associated with unsatisfactory post-operative outcomes.
Reference:
- Clin Ophthalmol. 2021 Jan 27;15:315-321.
Researchers Create Gene-Editing Technology to Eradicate Herpetic Stromal Keratitis
Shanghai researchers have developed a gene-editing method that rids the eye of keratitis, while eliminating the herpes simplex virus, reports an article in ShanghaiDaily.com . The method has been successful on both mice and donated human corneas.
Specifically, Dr. Hong Jiaxu, of the Shanghai Eye, Ear, Nose and Throat Hospital, and Cai Yujia, a Shanghai Joao Tong University researcher, joined forces to create a virus-like particle mRNA to “infect” the target and deliver HSV-1 Erasing Lentiviral Particles via dripping the technology into the infected patient’s eyes.
Diabetes Mellitus Predisposes Patients to Recurrent Corneal Erosion
Diabetes mellitus (DM) patients are at higher risk for recurrent corneal erosion (RCE), reports a recent study in the BMJ.1
Specifically, 1,236 DM patients vs. 884 control patients developed the painful condition. Additionally, in adjusting for potential confounders, such as chronic renal disease, hyperlipidemia, hypertension and keratoconjunctivitis sicca, the researchers discovered DM patients were 1.35 times more likely to develop RCE vs. the entire sample cohort.

The study was comprised of 239,854 DM patients who were recruited between 2003 and 2005 from the Longitudinal Cohort of Diabetes Patients database. The control patients were number, age and sex-matched and chosen from the Taiwan Longitudinal Health Insurance Database, 2000.
The study’s researchers say, “…Close collaboration between endocrinologists and ophthalmologists is important in managing RCE following DM.”
Reference:
- BMJ Open. 2020 Jun 21;10(6):e035933. doi: 10.1136/bmjopen-2019-035933.
Scleral Lens Wear Decreases Risk of Keratoplasty
Keratoconus (KC) patients who wear scleral contact lenses have close to 1/5 of the risk of undergoing keratoplasty, reports a recent study in Cornea.1
Specifically, out of 2,806 keratoconic eyes, 3.2% underwent keratoplasty, with scleral contact lens wear or rigid gas permeable contact lens wear significantly decreasing the need to undergo keratoplasty (HR = 0.19, 95% confidence interval [CI] 0.09-0.39, P < 0.0001 and HR = 0.30, 95% CI 0.17-0.52, P < 0.0001, respectively) vs. no contact lens wear.
Contact lens wear in each eye was 36.2% no wear, 7.2% soft contact lens wear, 33.9% rigid gas permeable wear and 22.7% scleral wear. Additional factors linked with a higher risk of keratoplasty were black race, younger age and lower socioeconomic status.
Given these findings, the study’s researchers say doctors should maximize the use of scleral or rigid gas permeable contact lenses on KC patients.
Reference:
- Cornea. 2021 Jan;40(1):39-42.
Atopic Keratoconjunctivitis Linked with Corneal Ulcer Risk
Patients who have atopic keratoconjunctivitis (AKC) are at an increased risk of developing a corneal ulcer, reports a recent British Journal of Opthalmology1 study.
Specifically, out of 171,019 newly diagnosed AKC patients, 2,018 developed a corneal ulcer, with the age-, potential comorbidities- and sex-matched control group comprised of 171,019 patients sans AKC. Both groups were tracked from January 2004 to December 2013.
Given this finding, the study’s researchers say patients who have AKC should be educated on their increased risk of developing a corneal ulcer.
Reference
- Br J Ophthalmol. 2020 Oct 3;bjophthalmol-2020-316206. doi: 10.1136/bjophthalmol-2020-316206. Online ahead of print.
Autophagy-Related 5 Potential Biomarker for Sjögren’s Syndrome
Atophagy-related 5 (ATG5) shows a 94.6% sensitivity and a 93.6% specificity (at a cutoff value of >4.0 ng/mL/μg) as an ocular biomarker for the diagnosis and assessment of Sjögren’s syndrome (SS), reports a study in Diagnostics.1
Specifically, the tear ATG5 concentration, among ocular surface disease index, ocular surface staining score, Schirmer’s test and tear break-up time were evaluated and compared in their ability to aid in differentiating SS from non-SS dry eye disease.
The study’s researchers say “the diagnostic power of tear ATG,” which other studies show is elevated in the conjunctival epithelial cells and tears of SS patients vs. non-SS dry eye disease patients, should be validated.
Reference:
- Diagnostics (Basel) 2021 2021 Jan 4;11(1):71.
ALSO NOTEWORTHY
- Aerie Pharmaceuticals, Inc. has announced the start of COMET-1, a Phase 2b clinical trial of AR-15512 (TRPM8 agonist) ophthalmic solution for dry eye disease (DED) treatment. The randomized, double-masked, vehicle-controlled trial will assess the efficacy and safety of AR-15512 in patients who have DED. A total of 360 patients are expected, with topline results expected in the third quarter of 2021. More information about the clinical trial is available at www.clinicaltrials.gov under the study designation NCT04498182.
- Alcon has announced the launch of AcrySof IQ Vivity, a non-diffractive extended depth of focus IOL that features the company’s proprietary X-WAVE technology to stretch and shift light without splitting it. This provides high-quality vision at distance and intermediate distances, while affording functional near vision. In other news, Alcon has announced the U.S. launch of the PRECISION1 for Astigmatism, daily disposable, silicone hydrogel contact lens, which settles in less than 60 seconds, provides 3º of orientation and features SMARTSURFACE Technology. This technology is a permanent micro-thin, layer of moisture that steps up from 51% water at the core, to greater than 80% water at the outer surface. For more information, visit www.Precision1.com .
- Azura Ophthalmics Ltd., an Israeli-based company with operations in the U.S. and Australia, has announced it has raised $20 million in a financing round, which will be used for the company’s lead product candidate AZR-MD-001 through a registration study as a treatment for meibomian gland dysfunction. AZR-MD-001 is a topical ointment for the lower lid that has shown a positive safety and efficacy profile. As a result of this and communication with the FDA, Azura says it plans to perform registration studies this year.
- Bausch + Lomb announced the SimplifEYE intraocular lens delivery system is available for the enVista MX60PL and the enVista toric MX60PT, a pre-loaded toric IOL. The delivery system allows for the implantation of enVista lenses into the capsular bag in three steps through an incision as small as 2.2 mm, and the system’s beveled tip enables consistent lens folding to provide surgeons with reproducible and reliable delivery of the enVista IOL into the capsular bag, the company says. Further, clear construction affords visualization of the IOL during implantation, and the proprietary coating technology of the inserter eliminates linear additive transfer deposits on the IOL surface post-implantation. Finally, a viscoelastic port ensures proper IOL lubrication that enables smooth passage of the IOL during delivery, according to Bausch + Lomb.
- BostonSight announced it has formed a scientific advisory board made up medical experts to help guide its research initiatives. The board: Sara Yost, BostonSight President and CEO; Dan Brocks, MD, chief medical officer; Demi Niforos, MS, vice president of Biostatistics and Statistical Programming at eClinical Solutions; Michael Raizman, MD, a practitioner at Ophthalmic Consultants of Boston and associate professor of Ophthalmology at Tufts University School of Medicine; Ali Djalilian, MD, professor of Ophthalmology, Cornea Service and Director of the Stem Cell Therapy and Corneal Tissue Engineering Laboratory at Illinois Eye and Ear Infirmary; and Gloria B. Chiu, OD, FAAO, FSLS, associate professor of Clinical Ophthalmology at the USC Roski Eye Institute, Department of Ophthalmology at the University of Southern California Keck School of Medicine.
- Bruder Healthcare Company LLC. announced it collaborated with ophthalmic surgeons and co-managing optometrists to create the Bruder Sx Pre-Surgical Patient Prep Kit, which is comprised of Bruder Hygienic Eyelid Cleansing Wipes; Bruder Hygienic Eyelid Solution; the Bruder Sx Pre-Surgical Compress; and the Bruder Sx Case. The Bruder Sx Pre-Surgical Patient Prep Kit is now available for in-office distribution or patient purchase by referral online. Call (888) 827-8337, or visit order.bruder.com .
- BVI has announced it has acquired Benz Research & Development, an independent supplier of performance materials and manufacturing technology to the IOL industry, and its customized contact lens subsidiary, SpecialEyes. BVI says Benz complements its portfolio of IOLs under the PhysIOL brand, which includes the trifocal lens FineVision.
- CooperVision Specialty EyeCare has named Marie-Christine Blanchard as global lead, Irregular Cornea, making her responsible for overseeing category growth across the company, including guiding cross-functional teams in support of the growing number of eye care professionals adopting technologies, such as scleral contact lenses.
- EyecareLive announced a licensing agreement with Allergan, an AbbVie company, to provide virtual consultations to those suffering from chronic dry eye disease symptoms. Patients will have access to an eye care provider who can recommend appropriate treatment, says Raj Ramchandani, CEO of EyecareLive. Visit www.eyecarelive.com/allergan .
- Fight for Sight and the Keratoconus Self-Help and Support Association are funding research into the development of a compact and portable lab-based laser tool to detect/monitor the slight biomechanical structural changes that take place during keratoconus to enable early detection.
- Johnson & Johnson Vision announced the FDA approval of the company’s TECNIS Eyhance and TECNIS Eyhance Toric II IOLs. TECNIS Eyhance combines low-light performance with breakthrough refractive surface design, providing a new kind of monofocal experience for cataract patients, says Rajesh K. Rajpal, MD, chief medical officer and global head of clinical and medical affairs at Johnson & Johnson Vision.
- Kala Pharmaceuticals, Inc. announced the launch of EYSUVIS (loteprednol etabonate ophthalmic suspension) 0.25% for the short-term (up to two weeks) treatment of the signs and symptoms of dry eye disease. The drug is available in national and regional U.S. pharmaceutical distribution centers. (See this month’s “Product Spotlight” for additional details on EYSUVIS.) See full prescribing information at www.eysuvis.com .
- Ocular Therapeutix announced the submission of a supplemental New Drug Application to the FDA for DEXTENZA (dexamethasone ophthalmic insert) 0.4 mg for intracanalicular use for the treatment of ocular itching associated with allergic conjunctivitis.
- Oculis SA named David Jacobs, MD, as chief development officer. Dr. Jacobs will be responsible for heading up all development plans and acquiring market authorizations by coordinating all regulatory activities required for future New Drug Application submissions to the FDA and the equivalent for regulators in other countries.
- OCULUS Optikgeräte GmbH has partnered with Myopia Profile to increase eye care practitioner knowledge and clinical confidence in measuring and interpreting axial length in myopia, via creating specific educational content, research summaries and case studies that will be available on www.myopiaprofile.com and shared across the partnership’s multiple platforms.
- Orasis Pharmaceuticals announced the initiation of Phase 3 clinical studies on its eye drop candidate created to improve near vision in presbyopia. The studies, which will be comprised of around 600 subjects in the U.S., will be the final stage of clinical development before potential commercial launch, the company says.
- Rowan University Henry M. Rowan College of Engineering researcher Dr. Iman Noshadi has won an award from Philadelphia’s University City Science Center’s Proof-of-Concept Program for the development of a strong, resorbable, nontoxic ocular sealant that can be used on wet ocular surfaces and, thus, makes it a great fit for corneal tissue repair and eye injuries, reports a press release from Rowan University.
- Vanda Pharmaceuticals Inc. has announced the FDA has approved its Investigational New Drug Application to assess Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) activator VSJ-110 (previously known as CFTRact-K267) for allergic conjunctivitis treatment. The initial Phase 2 study will assess the acute anti-inflammatory effects of VSJ-110 in an ocular allergic challenge model, and will assess the prosecretory effects, employing standard tear production assessments. “Initiation of the clinical program for VSJ-110 marks the beginning of Vanda’s development of therapeutics in ophthalmology exploring the compound’s novel dual anti-inflammatory and prosecretory mechanism of action,” says Mihael H. Polymeropoulos, MD, Vanda’s President and CEO.
- Visus Therapeutics, a clinical-stage company working on creating a presbyopia-correcting eye drop, has announced it has appointed Marguerite McDonald, MD, Neda Shamie, MD, William Trattler, MD, and Lawrence Woodard, MD, to its clinical advisory board. These ophthalmologists will use their knowledge base to provide strategic guidance related to the company’s clinical development program for BRIMOCHOL, an investigational eye drop for restoring the loss of near vision associated with presbyopia. Eric Donnenfeld, MD, leads the clinical advisory board. CP








