Because Descemet’s membrane endothelial keratoplasty (DMEK) tissue offers improved visual acuity, rapid visual rehabilitation, and a stable cornea, it is an attractive surgical option for treating symptomatic endothelial dystrophies, such as Fuchs’ endothelial dystrophy.1
Here, we provide tips on how to make DMEK efficient and predictable.
Pick the “Right” Patient
Some prior ocular surgeries take patients out of the running for DMEK in a surgeon’s early experience. For example, if a patient has a glaucoma drainage device, the fluid dynamics of the anterior chamber are less predictable, so it may be more difficult to unfold the DMEK graft in these patients. Additionally, post-vitrectomy, the anterior chamber will be deeper and difficult to shallow, as needed for ease of DMEK graft unfolding.2 This more difficult anatomy has been correlated with longer DMEK unfolding times for these eyes 3,4
Also, the postoperative course may be more challenging in the aforementioned patients. Specifically, the air bubble tamponade may be cleared too quickly by a glaucoma drainage device, or it may move into the posterior chamber in a vitrectomized eye. In such situations, the graft is more likely to detach and need re-bubbling. In some complicated cases, multiple re-bubbling may be required. As a result, Descemet’s stripping automated endothelial keratoplasty (DSAEK) with a thicker donor graft that is more easily unfolded and positioned against the recipient cornea, may be the preferred surgery for these types of complicated cases.
Something else to consider: DMEK is not always the best option for Fuchs’ endothelial dystrophy. If a patient has guttae concentrated in the central 4 mm of the cornea, functional vision in the fellow eye, and patience for prolonged visual rehabilitation, a Descemet’s stripping only (DSO) procedure may be a good option for the patient.5 This is especially the case for Fuchs’ endothelial dystrophy patients who may have contraindications to topical corticosteroids, cannot position supine during the postoperative period, or may have difficulty travelling to multiple follow-up appointments — steps all critical to the success of the DMEK procedure.
Plan Carefully
Efficient DMEK surgery requires careful planning regarding the size of the graft. Thus, a small DMEK graft may be needed based on the patient’s corneal size, location, and length of incisions (including from prior surgeries), peripheral scarring, or synechiae.
Many DMEK surgeons are now using preloaded DMEK tissue. This has the associated advantages of reducing the time needed to prepare the graft, and its safety has been well-reported, with no significant endothelial cell loss related to preloaded tissue storage or transportation.6,7 However, successfully using preloaded tissue requires the eye bank understand the surgeon’s preferences for sizing and tissue marking.
It’s worth noting that some graft-sizing limitations can become evident during surgery, so if the graft is precut, there is not the option to decrease the graft size. Additionally, if the DMEK graft is too large for the eye, it can be difficult to unscroll, it may overlap the incisions, and could prolong surgical time.
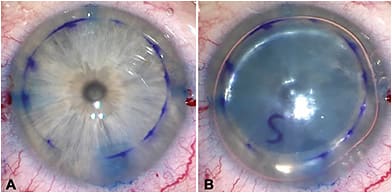
If the DMEK graft overlaps any of the incisions, insertion of balanced salt solution or air through those incisions will cause graft detachment. We have found that using trypan blue dye to stain the internal entry of the incisions prior to DMEK placement aids in the determination of optimal graft size, and acts as a guide for graft positioning (Figure 1). Trypan blue dye or air can also be used to facilitate visualization during the descemetorhexis.
Something else to plan for: cataract surgery. Specifically, if the patient also needs cataract extraction, consider staging the procedures. We typically perform phacoemulsification first, but will offer the DMEK first if the patient has significant corneal astigmatism, and is interested in achieving as much spectacle independence as possible with a toric IOL implant. This is because keratometry can change significantly after the cornea deturgesce post-DMEK.
If the cataract is mild, contemplate leaving the patient phakic and, instead, performing DMEK alone. Phakic DMEK is actually quite straightforward, well tolerated and, due to the curved anterior lens capsule, actually makes unfolding the graft easy. We have noted that cataracts typically do not progress rapidly despite the large postoperative air bubble.
Go With a Block
Consider performing a peribulbar or retrobulbar block for DMEK patients instead of using topical anesthesia. This allows for control of the globe, and an efficient operation without worrying about patient compliance and comfort.
Additionally, we feel that the block helps to minimize pressure on the cornea from lid squeezing after surgery. We prefer patients to have a secure patch (not a pressure patch) for the night after surgery to avoid compression of the corneal apex, potential gaping of the corneal wounds, and consequent graft separation.
Prevent Fibrin
Intraoperative fibrin will make it difficult to unfold and center the DMEK graft (Figure 2). Fibrin can result from excessive intraocular manipulation prior to inserting the DMEK graft, especially iris maneuvers with the risk of iris hemorrhage. Therefore, surgeons should perform an inferior laser peripheral iridotomy preoperatively, rather than risk fibrin accumulation with an intraoperative surgical iridectomy. Keep in mind that fibrin is also more likely in an inflamed eye, so it’s best to avoid performing DMEK in a “red” eye. We should always treat inflammation ahead of time, or alternatively perform a DSAEK if the inflammation is persistent.
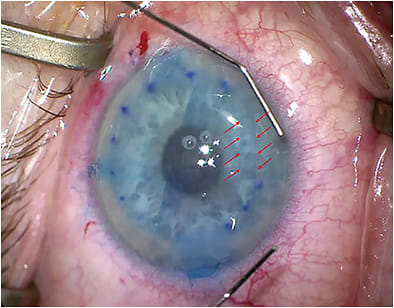
When encountering fibrin intra-operatively, surgeons should perform irrigation-aspiration immediately prior to inserting the DMEK graft, as the fibrin bands can hinder the unfolding of the graft. There are also reports of surgeons using intracameral tissue plasminogen activator (tPA) either prophylactically in cases that are suspected pro-inflammatory, or after fibrin has been observed.8 The tPA has been successful in diminishing or clearing fibrin from the anterior chamber.
Pre-Place Sutures
Surgeons should check the wound integrity prior to inserting the DMEK graft, and pre-place a 10-0 nylon corneal suture, if necessary. The suture is “looped out of the wound” prior to graft insertion, and can be quickly tied after insertion. This prevents shallowing of the anterior chamber during suture placement, and allows the anterior chamber to remain formed during DMEK tissue manipulation. Also, it is important to reduce the possibility of air leakage postoperatively, which can cause graft detachment.
Although we use an air bubble, 20% Sulfur Hexafluoride (SF6) gas can be used instead of air for a longer graft tamponade. The use of 20% SF6 gas has been shown to reduce the re-bubble rate, and does not have an associated effect or increased rate of pupillary block as compared to an air tamponade, as long as a patent inferior iridotomy is present.9,10
Unscroll the Tissue
Prior to inserting the DMEK graft, the tissue can be manipulated to produce a configuration that will be easier to open once in the eye. Preloaded tissue can be manipulated in an injector to create a double-scroll configuration, as described by Dr. Peter Veldman.11 Non-preloaded tissue can be manipulated while it is in a Petri dish (Figure 3). A Fogla DMEK cannula (which emits 2 streams of fluid perpendicular to the tip) can be an effective way to produce a double scroll in the Petri dish prior to loading the DMEK tissue. The Fogla DMEK cannula is also useful to have on hand for intraocular graft opening, if faced with a tight scroll.
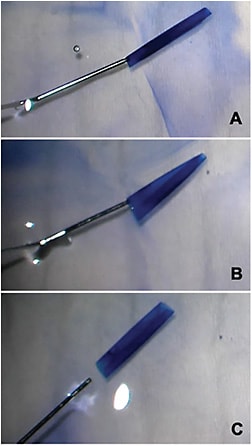
When performing “the DMEK dance,” keep the entire surgical picture in mind at all times — consider graft configuration, anterior chamber depth, and graft centration. Understand the various named configurations of the DMEK tissue, and the various methods that can be used for opening each configuration (see www.patientready.org ). Do not fully open the graft until it is well centered because it will be more difficult to center once it is open.
Additionally, we have discovered that it is critical to take the time to ensure that there are no Descemet’s membrane (DM) tags after DM stripping. The thin DMEK graft is less forgiving than a DSAEK graft, so if there are any remaining tags, the graft may not adhere.
We leave a large air bubble at the end of the procedure, and have the patient stay in the postoperative area supine for 1 to 2 hours. We then use the slit lamp to assess bubble size, iris position, and IOP. We are able to make any necessary adjustments to the size of the air bubble at this time, and then send the patient home with a secure patch. Other surgeons adjust the bubble size after a 10-minute wait time intraoperatively and ensure a freely mobile air bubble of about 8 mm in size. The air bubble, which is left in the eye, is just large enough to cover the edges of the DMEK graft, but small enough to not block the inferior peripheral iridotomy. The patient is counseled about the importance of supine positioning in the 72 hours following DMEK surgery.
A Completed Partial Thickness Procedure
Overall, DMEK can be a very rewarding procedure for both the surgeon and patient, with improved visual acuity outcomes, and faster visual rehabilitation. We recommend starting with less complicated cases, using older donor tissue (thought to unscroll more easily), if possible, early in the learning curve, and taking the time for strategic surgical planning. Doing so will likely result in a more efficient and effective surgery. CP
References:
- Dunker SL, Dickman MM, Wisse RPL, et al. Descemet Membrane Endothelial Keratoplasty Versus Ultrathin Descemet Stripping Automated Endothelial Keratoplasty: A Multicenter Randomized Controlled Clinical Trial. Ophthalmology. 2020;127(9):1152-1159.
- Weller JM, Tourtas T, Kruse FE. Feasibility and Outcome of Descemet Membrane Endothelial Keratoplasty in Complex Anterior Segment and Vitreous Disease. Cornea. 2015;34(11):1351-1357.
- Yamada N, Hayashi T, Yuda K, et al. Outcomes of Descemet Membrane Endothelial Keratoplasty for Vitrectomized Eyes With Sutured Posterior Chamber Intraocular Lens. J Ophthalmol. 2018;2018:3127126.
- Yoeruek E, Rubino G, Bayyoud T, Bartz-Schmidt K-U. Descemet membrane endothelial keratoplasty in vitrectomized eyes: clinical results. Cornea. 2015;34(1):1-5.
- Garcerant D, Hirnschall N, Toalster N, Zhu M, Wen L, Moloney G. Descemet’s stripping without endothelial keratoplasty. Curr Opin Ophthalmol. 2019;30(4):275-285.
- Zeidenweber DA, Tran KD, Sales CS, Wehrer SW, Straiko MD, Terry MA. Prestained and Preloaded DMEK Grafts: An Evaluation of Tissue Quality and Stain Retention. Cornea. 2017;36(11):1402-1407.
- Newman LR, DeMill DL, Zeidenweber DA, et al. Preloaded Descemet Membrane Endothelial Keratoplasty Donor Tissue: Surgical Technique and Early Clinical Results. Cornea. 2018;37(8):981-986.
- Ferguson TJ, Traboulsi EI, Goshe JM. Successful pediatric DMEK facilitated by intracameral tissue plasminogen activator to mitigate anterior chamber fibrin reaction. Am J Ophthalmol Case Rep. 2020;19:100812.
- Terry MA, Straiko MD, Veldman PB, et al. Standardized DMEK Technique: Reducing Complications Using Prestripped Tissue, Novel Glass Injector, and Sulfur Hexafluoride (SF6) Gas. Cornea. 2015;34(8):845-852.
- von Marchtaler PV, Weller JM, Kruse FE, Tourtas T. Air Versus Sulfur Hexafluoride Gas Tamponade in Descemet Membrane Endothelial Keratoplasty: A Fellow Eye Comparison. Cornea. 2018;37(1):15-19.
- Veldman P. Patient Ready DMEK. https://patientready.org . Accessed May 25, 2021.










