Phakic IOLs provide a safe and alternative treatment for myopia and myopic astigmatism in patients who may otherwise not be candidates for laser vision correction due to thin corneas, myopia too high for safe laser vision correction (LVC), or refractory dry eyes.1 Currently, two phakic IOLs are available in the U.S.: the Visian Implantable Collamer Lens (ICL) (STARR Surgical) and the Verisyse (Johnson & Johnson Vision, Inc).
The Visian ICL is a posterior chamber IOL FDA approved for the treatment of myopia ranging from -3 D to -16 D with reduction of myopia of up to -20 D. It is also available in a toric option that can treat a range of 1 D to 4 D of astigmatism.2,3 The Verisyse is an iris claw anterior chamber phakic IOL FDA approved to treat spherical myopia ranging from -5 D to -20 D with less than 2.5 D of astigmatism.4 As the underlying condition leading to the choice of phakic IOL is typically bilateral, most cases undergo bilateral implantation.
Here, we discuss these IOLs in terms of patient candidacy, successful implantation, postoperative care, and possible complications. (See “Phakic IOLs on the Horizon,” below.)
PHAKIC IOLS ON THE HORIZON
The EVO Visian ICL has several holes allowing for aqueous flow and eliminates the need for peripheral laser iridotomies. It is currently available in Europe, and is undergoing clinical trials in the United States. It has demonstrated IOP stability and a decreased rate of cataract formation, thought to be due to improved nutrition to the natural lens in addition to excellent visual outcomes.14
The EVO ICL for the treatment of hyperopia is currently available outside the U.S.
Additional phakic IOLs in the pipeline include a piggyback option for refractive errors, and a multifocal option in phakic and pseudophakic patients.
These exciting options on the horizon may provide alternatives for various patient types hoping to achieve their refractive goals.
Patient Candidacy
Phakic IOL patients must be evaluated carefully for surgical candidacy.
General inclusion criteria2
Age > 21 and < 45
- Iridocorneal angle > 30°
- Endothelial cell count > 2500 cell/mm2 if >21 years old; > 2000 if > 40 years old
- Mesopic pupil size < 6.0 mm
- Stable refraction
General exclusion criteria2,5:
- Active anterior segment pathology or uveitis
- Previous intraocular surgery
- Anterior chamber depth measured from endothelium < 3.0 mm for Visian and 2.7 mm for Verisyse
- Scotopic pupil diameter > 7.0 mm
- Cataract
- IOP > 21 mmHg or underlying glaucoma
- Macular pathology
- Zonulopathy
- Iris abnormalities (i.e., synechia or defect)
- Pregnancy or nursing
Successful Implantation
Phakic IOL implantation is a relatively seamless surgery as long as surgeons follow the appropriate steps. Success can be achieved via:
- Precise pre-operative measurements. These are necessary to both ensure patients are good candidates, and to choose the correct IOL size. The horizontal white-to-white (WTW) evaluation is a way to measure and predict the diameter of the ciliary sulcus, which determines the size of the IOL to be implanted. This can be measured using calipers, scanning slit topography, Scheimpflug photography, biometry, ultrasound biomicroscopy, or digital ultrasound.6 Surgeons typically develop their own system for WTW evaluation, given that these tools have significant variability and may often overestimate it.7 Surgeons may not use the same equipment for WTW measurement, and have to develop their own nomogram based on experience, occasionally deviating from the recommended on-label sizing.
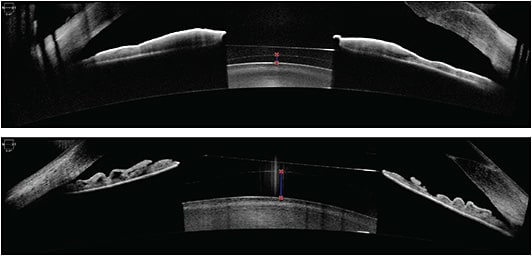
AS-OCT images depicting adequate vaults (250 μm and 740 μm, respectively) of an ICL over the natural crystalline lens.IMAGE COURTESY KATHRYN HATCH, MD
- Accurate anterior chamber depth measurement. This can be measured using biometry, Scheimpflug photography, AS-OCT, or partial coherence interferometry.8 Many biometers measure anterior chamber depth from the corneal surface to the lens, rather than from the endothelium to the lens, so the central corneal thickness must be subtracted from the measurement to arrive at an accurate measurement.
- Assessment of the patient’s refractive error and level of astigmatism pre-operatively. This can be achieved via using manifest refraction, and cycloplegic refraction. Corneal topography needs to be done to ensure regular astigmatism, with a 5 to 7 day contact lens holiday, and the management of dry eye disease, if present. (Repeat measurements to ensure accuracy may be necessary). If a patient does not have enough astigmatism to warrant a toric phakic IOL, limbal relaxing incisions can be considered intraoperatively or postoperatively. (The toric Verisyse option can treat 1 D to 7 D of astigmatism, but is not approved in the United States.9 Prior to the availability of the toric phakic IOL option, consideration was also given for LVC after phakic IOL insertion for residual visually significant refractive errors.
- Utilization of cohesive viscoelastic. This should be used, as it is easier to remove than dispersive viscoelastic.
- Prevention of pupillary block with the Visian ICL. Two large peripheral iridotomies, typically made at 10 o’clock and 2 o’clock, should be made preoperatively or intraoperatively to prevent pupillary block. Additionally, careful note should be taken of ideal ICL vault, typically measured relative to corneal thickness. Optimal vault is 0.75 to 1.5 corneal thickness on slit lamp examination, approximately 250 μm to 750 μm.10
- Prevention of pupillary block with the Verisyse IOL. The pupil should be constricted with pilocarpine prior to surgery. A large wound of 5.0 mm to 6.0 mm is necessary to allow IOL insertion. The wound size is chosen based on the size of the IOL, ranging in optic sizes of 5.0 mm and 6.0 mm. While the 6.0 mm optic is preferred to help avoid glare in scotopic conditions, in higher powers the lens thickness is such that it cannot be safely implanted, as it increases the risk for endothelial damage in shallower anterior chambers.11 In the higher powers, the smaller optic is often chosen. Care should be taken not to dilate the pupil when injecting viscoelastic, and not to place any under the iris, nor push the iris posteriorly, as these seemingly minor errors in surgical technique could impede the ability to enclave the iris with the Verisyse IOL.12
Postop Care
In the acute postoperative phase, IOP and sizing should be evaluated. Often, surgeons check the IOP 30 minutes prior to patient discharge, as acute postoperative IOP spikes can be related to retained ophthalmic viscosurgical devices. IOL vault is typically assessed in the office often a few hours after surgery, as well as the next day. Proper vault of approximately 0.75 to 1.5 central corneal thickness suggests appropriate sizing.
The close monitoring of the vault and the IOP must continue until the pupil size normalizes to a size smaller than the outer edge of the phakic IOL. This allows for a more accurate assessment of the sizing of the IOL, the stabilized vault, and the patency of the peripheral iridotomy (PI). If the IOP rises above normal when the pupil size normalizes, the PI patency should be evaluated and corrected if not patent. If the IOP rises and the PI appears patent, one must assess for angle closure, due to a large phakic IOL, by evaluating the vault and the angle structures. The patient should be dilated, and the vault reassessed. If dilation leads to a lessened vault, one could consider an insufficient PI or retained viscoelastic behind the IOL. If the vault does not change, one must consider an improperly sized IOL and consider exchanging the lens with a smaller IOL.
Long-term postoperatively, IOP and endothelial cell count should be followed at least annually for routine cases, and with concerns, including borderline vault or IOP, more frequently, such as every 3 to 4 months, to ensure there are no postoperative complications, such as endothelial cell loss and glaucoma. If there is residual astigmatism postoperatively, LRIs or laser vision correction can be considered, depending on the patients’ candidacy, and level of visual significance.

Possible Complications
Possible complications, which are relatively uncommon from phakic IOLs, are cataract formation, pupillary block glaucoma, endothelial decompensation, inflammation, wound leak, retinal detachment, and a small risk of endophthalmitis and/or bleeding.12 The incidence of cataractogenesis has decreased with the newer ICL models due to improved vaulting.11
Low vault with the Visian ICL is likely more associated with cataract formation.11 Endothelial cell loss is more common with the Verisyse IOL, given its placement in the anterior chamber.11 The minimum distance between the corneal endothelium and the Verisyse should be 1.5 mm to decrease the risk of decompensation, as the optic vaults 0.87 mm anterior to the iris.11,13
Additionally, significant surgically induced astigmatism can result in decreased, uncorrected postoperative visual acuity. Refractive surgical procedures, such as LRIs or laser vision correction, may need to be considered.
Also, glare and haloes can be debilitating to patients postoperatively. This can occur due to edge effects of the optic size of the IOL in relation to pupil diameter, if the patient’s pupil size is not carefully evaluated preoperatively. If the scotopic pupil size is larger than the optic of the phakic IOL, a patient can experience more glare postoperatively.11 The Visian ICL has an optic diameter of up to 5.5 mm. The Verisyse has an optic diameter of up to 6.0 mm. Iris retraction and pupil ovalization can occur in the Verisyse IOL if the fixation of the haptics is asymmetric, which can lead to optical aberrations if the IOL is not centered over the pupil center.11
Further, decentration and/or rotation of the IOL can occur if it is not sized appropriately, which is why horizontal WTW evaluation is critically necessary.11 Additionally, if the Visian ICL is oversized, there is a small risk of high vault and uncontrolled IOP requiring IOL exchange.
A novice surgeon may want to operate on one eye at a time until his/her sizing algorithm is established. CP
References:
- Güell JL, Morral M, Gris O, Gaytan J, Sisquela M, Manero F. Five-year follow-up of 399 phakic Artisan-Verisyse implantation for myopia, hyperopia, and/or astigmatism. Ophthalmology. 2008;115(6):1002-1012.
- Güell JL, Morral M, Kook D, Kohnen T. Phakic intraocular lenses: part 1: historical overview, current models, selection criteria, and surgical techniques. J Cataract Refract Surg. 2010;36(11):1976-1993.
- STAAR Surgical. Vision ICL. STAAR Surgical’s phakic IOL for myopia, hyperopia and astigmatism. https://staar.com/products/visian-icl . Accessed May 18, 2021.
- Ophtec. WHAT ABOUT THE VERISYSE? The ARTISAN Myopia lens and the Verisyse lens are the same device manufactured by OPHTEC in Groningen, the Netherlands. https://usa.ophtec.com/products/verisyset . Accessed May 18, 2021.
- Pérez-Cambrodí RJ, Piñero DP, Ferrer-Blasco T, Cerviño A, Brautaset R. The posterior chamber phakic refractive lens (PRL): a review. Eye (Lond). 2013;27(1):14-21.
- Hasan S, Tripathy K. Phakic intraocular lens myopia. In: StatPearls. StatPearls Publishing; 2021. www.ncbi.nlm.nih.gov/books/NBK560763/ . Accessed April 18, 2021.
- Vaitheeswaran LG, Hemamalini MS, Kurian M, Shetty R. Comparison of ICL sizing using WTW (white to white) and ACD (anterior chamber depth) measurements from different devices. In: Chakrabarti A. Proceedings of 76th Annual Conference of All India Ophthalmological Society. All India Ophthalmological Society; 2018:236-243.
- Chang DH, Davis EA. Phakic intraocular lenses. Curr Opin Ophthalmol. 2006;17(1):99-104.
- Alió JL, Toffaha BT. Refractive surgery with phakic intraocular lenses: an update. Int Ophthalmol Clin. 2013;53(1):91-110
- Alfonso JF, Fernández-Vega L, Lisa C, Fernandes P, Jorge J, Micó RM. Central vault after phakic intraocular lens implantation: Correlation with anterior chamber depth, white-to-white distance, spherical equivalent, and patient age. J Cataract Refract Surg. 2012;38(1):46-53.
- Kohnen T, Kook D, Morral M, Güell JL. Phakic intraocular lenses: part 2: results and complications. J Cataract Refract Surg. 2010;36(12):2168-2194.
- Hardten DR. Phakic iris claw artisan intraocular lens for correction of high myopia and hyperopia. Int Ophthalmol Clin. 2000;40(3):209-221.
- Tinwala SI. Phakic Intraocular Lenses An Overview. Delhi J Ophthalmol. 2013;24(1):7-15.
- Packer M. The Implantable Collamer Lens with a central port: review of the literature. Clin Ophthalmol. 2018;27(12):2427-2438.










