We are fortunate to have an ever-expanding list of dry eye disease (DED) treatments to help the > 16 million U.S. adults who have diagnosed DED.1 The latest therapeutic advances are in-office treatments designed for patients unable to achieve symptomatic relief via at-home treatments, such as artificial tears, alone. It is rewarding to be able to offer these patients further treatments in the office to help alleviate their battle with DED.
Here, I discuss the current in-office procedures available, in alphabetical order, and their specific benefit to our DED patients.
Amniotic Membrane
With the understanding that inflammation, including T cell migration and cytokine release, are features of DED, placement of amniotic tissue over the cornea is shown to help significantly reduce severe DED symptoms by decreasing surface inflammation, and providing a bandage-like effect by its placement on the corneal surface.
Amniotic membrane contains basement membrane and an avascular stromal matrix that is similar in composition to the conjunctiva. This, in theory, promotes better healing of the corneal epithelial cells and limbal stem cells, while preventing scar and vascular formation.2 The DREAM study, by McDonald et al, on amniotic membrane efficacy in DED, revealed 88% of patients had an improved ocular surface, as well as a notable decrease of severity, given the overall Tear Film & Ocular Surface Society Dry Eye Workshop score significantly dropped from 3.25±0.5 at baseline to 1.44±0.6 at 1 week, 1.45±0.6 at 1 month, and 1.47±0.6 at 3 months (p < 0.001).3
There are two main options for amniotic tissue at this time: cryo-preserved amniotic membrane (CAM) and dehydrated amniotic membrane. The risk of amniotic membranes are blurred vision, induced by the covering of the cornea, and mild discomfort.4
Placement of either type is straight forward. The CAM version typically requires a tape tarsorrhaphy to help partially close the lid, so that the device does not fall out, as the tissue is attached to a soft ring that keeps it properly positioned. The procedure typically takes about 5 to 10 minutes, not including patient counseling or assessment. This also allows the patient to partially open and close their eye, so that they can still use eye drops, if needed.
The dehydrated version is placed directly on the cornea, and then a bandage contact lens is placed over the membrane to keep it in the proper position. The patient is able to fully open and close their eye. The procedure typically takes about 5 to 10 minutes, and patients can return to their normal activities immediately.
It is important to counsel patients using either membrane that their vision will be reduced while the membrane is in place in that eye.
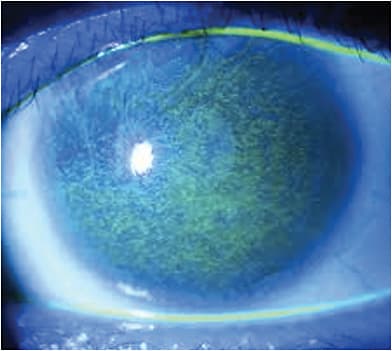
Autologous Serum Tears (AS)
In more severe cases of refractory DED, such as Sjögren’s syndrome or graft-versus-host disease, autologous serum tears (AS) can provide benefit.
The theory behind serum tear use is that the biochemical components of serum more closely mimic our own natural tears. Currently, no clinical formulations of AS exist, so they must be compounded with autologous serum.
Blood is drawn from the patient, and then centrifuged in a lab to separate the serum from the solid components. The resulting serum can then be diluted to the desired concentration.
A Cochrane Database review yielded many articles on the use of AS for DED, and showed some benefit of 20% autologous serum tears in treating DED for up to two weeks, but longer periods of follow-up provided no evidence of improvement in longer periods. Further large scale, randomized controlled clinical trials are needed for further evaluation.5
Intense Pulsed Light (IPL)
There has been a noted association between the chronic inflammatory changes of meibomian gland dysfunction (MGD) and skin rosacea, with the underlying pathology related to telangiectasias and concomitant inflammation from inflammatory mediators.6
There are many proposed mechanisms of action for IPL’s efficacy on MGD, including the thrombosis of the abnormal blood vessels at the skin’s surface, heating of the meibum (which allows for more effective secretion of the glands), reduction of epithelial cell turnover, improvement in collagen synthesis, and eradication of Demodex folliculorum mites, thought to be a causative agent in blepharitis and MGD.7 Many clinical studies showed IPL effective and safe as an adjuvant treatment.7 Additionally, IPL’s results can be maintained via periodic treatments.7
IPL employs a high-output flashlamp that passes a burst of electrical current through a xenon gas-filled chamber, and creates a broad wavelength of non-coherent light between 500 mm to 1,200 mm. It enables the treatment of specific tissues, while sparing others, and is non-invasive, making it a well-accepted treatment among patients.
Risks include blistering, skin burns and unwanted hair loss. Also, patients who have a recent history of using photosensitive medications or blood thinners do not make good candidates. Clinicians should also become familiar with the different Fitzpatrick Skin Types, as darker skin pigmentation cannot tolerate IPL, and have a risk of depigmentation.6
Some examples of IPL devices include the M22 (Lumenis), the Quadra Q4 Platinum Series IPL and, most recently, OptiLight (Lumenis), which is an FDA-approved IPL treatment for DED management. A multicenter, double-masked randomized controlled trial showed the device significantly improved tear break-up time (TBUT), meibum quality, and meibomian gland expressability.8 The device uses a patented handpiece to apply the treatment in a 15-minute session. Typically, 4 separate sessions are recommended and, often, a treatment is followed by meibomian gland expression. In addition to helping improve a patient’s DED signs and symptoms, patients also often receive an improvement in the appearance of their skin in the treated areas, thereby providing a cosmetic benefit as well.
Microblepharoexfoliation
The purpose of microblepharoexfoliation is to debride the eyelashes and lid margin in patients who have MGD and/or blepharitis to provide symptomatic relief. A recent ASCRS presentation showed that the in-office microblepharoexfoliation device BlephEx (Alcon) changed the average Standardized Patient Evaluation of Eye Dryness (SPEED) score from 16.65 to 6.85 (58% decrease: P < 0.05), the Ocular Surface Disease Index (OSDI) score from 62.50 to 29.25 (53% decrease; P < 0.05), and improved the average TBUT from 10.1 to 16.8 seconds (66% increase; P < 0.05) sans complications.
BlephEx consists of a handpiece that attaches to a single-use medical grade sponge. The handpiece rotates the sponge, so that when the sponge tip (after being soaked in a lid cleanser) is gently placed along the lid margin, it quickly debrides the eyelashes and lid margin.
The risks of BlephEx are a tickling sensation during the procedure (numbing drops are placed prior to the device’s use), and possible red or irritated lids, which subside within a day.
All four lids can be treated in about 6 to 8 minutes, and patients can resume their normal activities immediately afterward. Clinicians typically recommend BlephEx be performed 1 to 2 times per year to enable patients to maintain symptomatic relief. BlephEx treatments are often paired with thermal procedures, such as LipiFlow (Johnson & Johnson Vision), Systane iLux (Alcon), or TearCare (Sight Sciences) treatments.
Doctors may also want to prescribe the NuLids System (NuSight Medical). NuLids is an at-home treatment, used for 60 seconds a day, 15 seconds per lid, to massage the lids to remove collarettes, and unclog meibomian glands. (See www.nusightmedical.com .)
Ophthalmic Inserts
A way to provide continuous lubrication to the ocular surface without having to place artificial tears is the hydroxypropyl cellulose ophthalmic insert, also known as Lacrisert (Bausch + Lomb). The insert is placed in the inferior fornix by the patient, typically once daily, and provides preservative-free lubrication. It is indicated for the treatment of moderate-to-severe DED, including keratonjunctivitis sicca.
According to a multi-center clinical trial by McDonald et al., Lacrisert significantly reduced symptoms and signs of moderate-to-severe DED, and patients also experienced a statistically significant improvement in quality of life , as measured by the OSDI, of 21.3%.9
Adverse reactions include transient blurring of vision; ocular discomfort or irritation; matting or stickiness of eyelashes; photophobia; hypersensitivity; eyelid edema and hyperemia.10
Punctal Occlusion
Punctal occlusion has been a staple of DED management for many years now, as it is shown to elevate the tear layer to help provide a more constant lubrication to the ocular surface. Data indicate plugs can provide symptomatic relief from severe DED.11
The type of punctal occlusion ranges from placing permanent (silicone) or temporary (dissolvable) punctal plugs, to punctal cautery.
Permanent plugs typically have a longer duration of use (they should remain in place until they fall out or are removed), and allow the physician to easily ascertain whether they are still in place. For patients who feel these plugs when moving or blinking their eyes, a temporary (dissolvable) plug that can last up to 180 days may be prudent.
For patients who cannot achieve relief with either plug, it makes sense to investigate punctal cautery, or permanently closing the duct, via heat, to keep the tears on the ocular surface.
The adverse reactions associated with punctal plugs are watery eyes; improper fit; tear duct irritation, and, rarely, dacryocystitis. Patients should be educated to reach out to their doctor, should they experience any issues.
The procedure itself can be typically performed in about 5 minutes (not including patient assessment or counseling) during the patient’s visit and, therefore, does not add significant strain to the daily workflow. Patients can return to their normal activities immediately.
Thermal Treatment
In-office thermal procedures are shown to fill in the treatment gap for patients who have MGD, and are unable to gain symptomatic relief from at-home warm compresses and lid hygiene. A study revealed that combining thermal application with warm compress treatments resulted in significantly greater improvement in gland function [mean difference (MD): 2.19 (95% confidence interval (CI), 0.95, 3.43)], TBUT [MD: 1.08 (95% CI, 0.06, 2.10)], and SPEED scores [MD: -2.76 (95% CI, -4.22, -1.30)] at 2 to 4 weeks versus in patients who used warm compresses alone.12 Additionally, a significantly greater reduction in the OSDI was noted at 2 to 4 weeks [MD: -8.61 (95% CI, -13.62, -3.61)], and 3 months [MD: -6.92 (95% CI, -11.95, -1.89)] in the combination group vs. the warm compress group.12
Risks can include burning, corneal abrasion, eyelid/eye pain, foreign body sensation, inflammation, itching, redness, red eyes, tearing, and discharge, though these minor complications are quite rare.
The three thermal treatments available and their procedures:
- LipiFlow. LipiFlow has activators that go directly into the eye, and surround the anterior and posterior lid. Once the activators have been placed, the device performs the entire treatment automatically, including heating the meibum, allowing for gland expression with a pulsation force. The treatment takes 12 minutes.
- Systane iLux. Systane iLux combines heating and gland expression into one step and handheld device, while allowing the clinician to monitor, and control the entire procedure through a built-in magnifier lens. The device uses an applicator, which employs infrared energy to warm the glands. The treatment takes about 8 minutes.
- TearCare. TearCare has two steps. The first step is to place its proprietary activators external to the lids. The activators connect to a hub that provides a steady flow of heat to melt the meibum for 15 minutes. The heating device is then removed, and gland expression is performed manually with provided gland expression forceps.
Thermal treatment can be repeated as needed, but I often recommend every 6 to 12 months, depending on the patient’s severity of MGD and DED symptoms. Each procedure allows the patient to return to their normal activities with minimal, if any, downtime. I do recommend that patients resume their current regimen of daily warm compresses and lid hygiene afterwards to best maintain their treatment effect.
Making a Dent in DED
DED remains a huge problem, and we have still not discovered a single perfect treatment for every patient. However, we are fortunate now to have a variety of ways to help our patients in our offices. By continuing to address the multi-factorial nature of DED, from lack of tear production, to inflammation on the ocular surface and degeneration of the meibomian glands, we can continue to help our patients’ acute symptoms, and prevent further damage of their ocular surface. CP
References:
- Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am J Ophthalmol. 2017 Oct;182:90-98.
- Fukada et al. Differential Distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea and conjunctiva. Cornea 1999; 18:73-79.
- McDonald MB, Sheha H, Tighe S, et al. Treatment outcomes in the Dry Eye Amniotic Membrane (DREAM) study. Clin Ophthalmol. 2018 Apr 9;12:677-681.
- American Academy of Ophthalmology. Ophthalmic Pearls. In-Office Use of Amniotic Membrane. https://www.aao.org/eyenet/article/in-office-use-of-amniotic-membrane . Accessed July 19, 2021.
- Pan Q, Angelina A, Marrone M, Stark WJ, Akpek EK. Autologous serum eye drops for dry eye. Cochrane Database Syst Rev. 2017(2): CD009327.
- Toyos R, McGill W, Briscoe D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction: a 3-year retrospective study. Photomed Laser Surg. 2015;33(1):41–46.
- Dell SJ. Intense pulsed light for evaporative dry eye disease. Clin Ophthalmol. 2017 Jun 20;11:1167-1173.
- Giannaccare G, Taroni L, Senni C, Scorcia V. Intense Pulsed Light Therapy In The Treatment Of Meibomian Gland Dysfunction: Current Perspectives. Clin Optom (Auckl). 2019 Oct 17;11:113-126.
- McDonald M, D’Aversa G, Perry HD, Wittpenn JR, Donnenfeld ED, Nelinson DS. Hydroxypropyl cellulose ophthalmic inserts (lacrisert) reduce the signs and symptoms of dry eye syndrome and improve patient quality of life. Trans Am Ophthalmol Soc. 2009;107:214-21.
- Lacrisert Prescribing Information. https://www.lacrisert.com/siteassets/pdf/Lacrisert-package-insert.pdf?ver=2017-01-04-093619-360 . Accessed September 24, 2021.
- Ervin AM, Wojciechowski R, Schein O. Punctal occlusion for dry eye syndrome. Cochrane Database Syst Rev. 2010;(9):CD006775.
- Pang SP, Chen YT, Tam KW, Lin IC, Loh EW. Efficacy of Vectored Thermal Pulsation and Warm Compress Treatments in Meibomian Gland Dysfunction: A Meta-Analysis of Randomized Controlled Trials. Cornea. 2019 Jun;38(6):690-697.









