Microbial keratitis (MK) presents a challenge in identification, treatment, and visual outcomes. Several types of infection are more common in the cornea, including bacterial and viral infections. That said, atypical corneal infections may occur, leading even the most experienced corneal specialist astray from the true diagnosis.
This article describes the characteristics of atypical cornea infections, actions in regard to evaluation and workup, and tips for the treatment and management of these difficult cases.
Characteristics
The following are examples of atypical organisms and their characteristics:
• Acanthamoeba keratitis (AK). This often presents clinically like herpes simplex virus (HSV) keratitis. Specifically, it includes punctate epithelial defects, dendrite-like staining, a ring infiltrate, endothelial plaque, corneal thinning as well as haze, or infiltrate along the nerves. Additionally, some patients may develop focal or nodular scleritis, which has a poor visual prognosis. The key symptom is pain out of proportion to the findings, resulting from the perineuritis. Due to misdiagnosis, often these patients are treated with a topical steroid, which then worsens the visual prognosis.
• Microsporidia keratitis. Microsporidia are intracellular protozoa that can infect the cornea. It can affect the stroma in immunocompetent eyes and appear more superficially in immunosuppressed patients. Microsporidia appear as smoldering, diffuse, multifocal stromal lesions, with lid edema and a congested conjunctiva1 (Figure 1). Patients report recurrent redness, pain, photophobia, watering, and reduced vision. This can be mistaken for HSV keratitis.
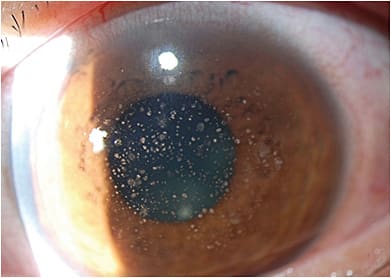
Regarding patient history, contact lens wear, exposure to contaminated water or soil, LASIK surgery, prior use of a topical corticosteroid, and trauma are associated with microsporidia keratitis.2
When HSV keratitis is suspected, we should ask patients whether they have a history of cold sores, hypoesthesia, and recurrent unilateral eye infections. (Past dendritic or geographic epithelial defects are characteristic of herpetic keratitis.) Doing so aids us in arriving at the correct diagnosis.
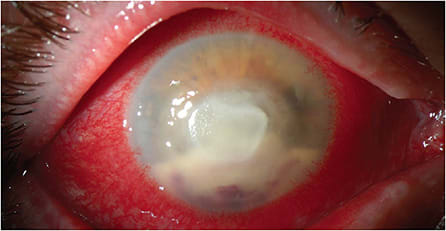
• Fungal keratitis. These atypical corneal infections are often indolent in nature. They can have less inflammation with less edema and injection initially versus all other forms of keratitis. Fungal keratitis may present clinically with an infiltrate without an epithelial defect for some patients. Red flags for this atypical form of keratitis are the presence of an infiltrate that has irregular feathery edges, an endothelial plaque, or the presence of a satellite lesion. Candida, as an example, often presents as a superficial, white-raised lesion (Figure 2). Hyphae indicate filamentous fungi, like Aspergillus or Fusarium (Figure 3).

Action Steps
The action steps regarding evaluation and workup of suspected atypical corneal infections are as follows:
• Evaluation. As with any keratitis, a careful patient history and slit lamp examination are warranted. Regarding the former, patients should be questioned about the timing and progression of infection symptoms. (See Fungal keratitis above.) Additionally, patients should be questioned about risk factors, such as contact lens wear, exposure to contaminated water or soil, steroid eye drop use, and immune-compromised status. In terms of patient history, Acanthamoeba keratitis (AK) is often associated with contact lens use or exposure to water or soil.
Further, a review of prior failed treatment is important to identify the keratitis. When common treatment regimens, such as fortified broad-spectrum antibiotics, work initially and then plateau, don’t work at all, or work initially and then worsen the keratitis, we should consider atypical causes of the keratitis.
Something else to keep in mind: As AK often presents clinically similar to HSV keratitis with a pseudodendrite or nonspecific epithelial defect, one must be careful prior to initiating topical steroids, as atypical infections may worsen with steroids. If a patient worsens upon addition of steroid, then the steroid should be stopped and workup initiated for atypical ulcer.
• Workup. Often, culturing will provide an answer as to what organism we are treating. However, atypical infections are notoriously difficult to grow on cultures. (Ways to overcome this are discussed below.)
When a patient presents with a nonhealing keratitis, I follow these steps:
- Determine which treatments helped versus not.
- Suggest a drug holiday for 48 hours, as long as the patient is not about to perforate.
- Culture using Gram stain slides, chocolate, blood, Lowenstein-Jensen, Sabaroud’s media or fungal slant, charcoal plate, and thioglycerate. For a deep ulcer, a braided 8-0 vicryl suture can be used to pass through the area of ulcer and sent for cultures.Keratitis suspicious for AK should be cultured on nonnutrient agar with an E. coli or Enterobacter aerogenes overlay, or a buffered charcoal-yeast extract agar. At our lab, scrapings on a Page’s saline plate can be used, and the lab transfers it to the above agars. Culturing or sending the contact lens and case for pathology is useful as well.
In my practice, a 2 mm to 3 mm cornea biopsy and confocal microscopy can also be helpful for diagnosis. Biopsy can be particularly useful for AK, especially since AK is tough to grow on culture.
Ulcers suspicious for microsporidia should be cultured using stains such as Giemsa, 1% acid fast or Brown-Hopps stain, or by transmission electron microscopy.3 Cultures are difficult and rarely show growth. However, microscopic examination of the epithelium or stroma can reveal the organism, especially with the use of KOH wet mounts and electron microscopy.3 (It makes sense to alert the pathologist to keep in mind that the differential includes microsporidia.)
Fungal suspicion warrants Sabouraud’s media. It may take days or weeks to identify. Negative cultures in the setting of possible fungal infection may be diagnosed with biopsy. - Consider doing a cornea biopsy if suspicion is high that the patient has an organism difficult to grow. (The cornea biopsy can also be done later if the cultures are not conclusive.) Performing the biopsy at a slit lamp is ideal because we can see the depth of the biopsy; however, some patients are not cooperative enough for the slit lamp, so they may need to be horizontal, while we use a microscope. (See “Key Steps to a Cornea Biopsy”.)
In some cases, the cultures, biopsy, and noninvasive imaging will be negative. In these cases, a large biopsy or a PK may be needed for pathology diagnosis. In addition, recalcitrant cases may need infection debulking to improve. - Contemplate utilizing noninvasive imaging. Confocal microscopy can be used to evaluate the keratitis for infection. Fungal infections, for example, can show septate hyphae or non-septate hyphae. Acanthamoeba cysts can be seen as well. It takes a skilled imager to obtain quality confocal images, especially when the keratitis is located more superficially
Tips for Treatment
Atypical corneal infections can be treated with various medications (options abound) and surgery:
• Medications. The first line treatment for AK in our practice is chlorhexidine gluconate 0.02% and propamidine isetionate 0.1% (Brolene, Sanofi-Aventis). Voriconazole 1% and polyhexamethylene biguanide can be used as well. The challenge is that once treated, some trophocytes will convert into cysts, which are difficult to eliminate. In addition, the toxicity of the drops may result in medicamentosa, neurotrophic epithelial defects, and intolerance from the patient. Toxicity, obviously, must be addressed. Treatment can range from months to even a year. Oral miltefosine (Impavido, Profounda, Inc.) has been used more recently in refractory cases of AK.
Treatment options for microsporidia keratitis include debridement, topical voriconazole 1%, and propamidine isetionate 0.1% (Brolene, Sanofi-Aventis). In addition, topical monotherapy with a fourth-generation fluoroquinolone has been reported to work.
For fungal keratitis, such as Candida, topical amphotericin 0.15% to 0.30% can be prescribed. For other fungal ulcers, topical natamycin 5% (Natacyn, Santen) is the first-line treatment. This is especially true for the filamentous fungi, like Fusarium. Topical natamycin q1h to q2h hours may work as monotherapy. Topical voriconazole 1% may be added if the ulcer is not responsive. The addition of moxifloxacin 0.5% may be synergistic, which may be why some ulcers are delayed in diagnosis as they improve with initial treatment of moxifloxacin.4
For deep subconjunctiva, or intra-stromal ulcers, voriconazole may be prescribed. In our practice, we utilize a diamond burr to perform a superficial keratectomy, then use a natamycin or voriconazole-soaked wick to help the drug penetrate. Oral voriconazole may also help, especially for Fusarium ulcers, as per the Mycotic Ulcer Treatment Trial.5 Dosing and duration are dependent on clinical improvement and the balance between toxicity and anti-infective treatment. It can range from weeks to months.
• Surgery. In a perfect case, one would hold off on surgery, especially a PK, until the infection is cleared. However, in the process of treating atypical ulcers, the cornea may start to thin and perforate. In our practice, we have found it helpful to take the patient to the operating room prior to perforation when the cornea is very thin to provide a multilayered amniotic membrane, injection of antimicrobials in a controlled setting, and even tarsorraphy to allow for surface healing. A biopsy can be done at the same time if the diagnosis is still unknown. This often postpones the need for more invasive treatment in the setting of active disease.
If a perforation is small in the setting of active disease, cornea glue, such as cyanoacrylate, can be used to postpone the need once again for emergent grafting. This allows for some quieting of the eye, and the glue may have antimicrobial properties. More importantly, this allows for scheduling with adequate tissue availability and operating room staff.
At the time of a keratoplasty in the setting of perforation, care should be taken not to seed organisms. As a result, I often have two sets of instruments. It is best to perform a PK larger than the infection; if that is not possible because it is limbal-involving, we should consider intrastromal injection in the recipient.
I do not put the patient on a steroid following PK when I do not know the infection. Instead, I use cyclosporine 1% q.i.d., and start a steroid once the pathologist has informed me the tissue has no infection, or that the organism was not in the peripheral margins of the donor button. Cyclosporine dosing ranges based on clinical picture. If the infection is still active, I continue with oral, injections and topical treatment until I feel it is sterile. The graft likely will fail without the steroid, but it’s essential to avoid it in active infection.
Overcoming the Challenge
Atypical corneal infections present a challenge to all clinicians, even the most seasoned ulcer specialist. Counseling patients about their challenging disease is important. An awareness of atypical ulcer characteristics, the steps to work them up, and treatment options that may include surgery will assist in managing these tough cases. CP
References:
- Alkatan HM, Al-Zaaidi S, Athmanathan S. Microsporidial keratitis: literature review and report of 2 cases in a tertiary eye care center. Saudi J Ophthalmol. 2012; 26(2):199–203.
- Kwok AKH, Tong JMK, Tang BSF, Poon RWS, Li WWT, Yeun KY. Outbreak of microsporidial keratoconjunctivitis with rugby sport due to soil exposure. Eye. (Lond). 2013;27(6):747-754.
- Joveeta J, Sridhar MS, Murthy S, Sharma S. Clinical and microbiological profile of microsporidial keratoconjunctivitis in southern India. Ophthalmology. 2006;113(4):531-537.
- Matoba AY. Fungal keratitis responsive to moxifloxacin monotherapy. Cornea. 2012;31(10):1206-1209.
- Prajna NV, Krishnan T, Rajaraman R, et al. Adjunctive oral voriconazole treatment of fusarium keratitis: a secondary analysis from the Mycotic Ulcer Treatment Trial II. JAMA Ophthalmol. 2017;135(6):520-525.









