Residual astigmatism after refractive cataract surgery impacts patients’ uncorrected visual quality, as well as their satisfaction with the procedure, especially in those receiving multifocal IOLs.1 Femtosecond laser-created arcuate incisions (AI) reduce mild-to-moderate astigmatism, enabling patients to achieve optimal visual outcomes from premium IOLs, such as multifocals.
Specifically, in a retrospective case study of 189 eyes that had a range of astigmatism of 0.5 D to 2.0 D, my colleagues and I found that femtosecond laser-assisted arcuate incisions significantly decreased astigmatism from 0.92 ± 0.34 D to 0.14 D ± 0.23 D (P < .001) with an optimized nomogram, and 95.8% of eyes had postoperative astigmatism of 0.5 D or less.2 Additionally, postoperative uncorrected distance acuity was 20/30 or better in 90% of eyes, and postoperative results remained stable for at least 1 year.
I prefer femtosecond arcuate incisions over manual arcuate incisions for several reasons. First, I find that the femtosecond laser can place the incisions more precisely. Second, the reproducibility of the incision is enhanced by the laser’s precision. Consequently, having an accurate process every time with minimal variability in the length, depth, and morphology for a specific incisional treatment should provide superior outcomes. Lastly, these outcomes would be more titratable, allowing for meaningful nomogram refinement.
Here, I discuss the steps surgeons must take to optimize the visual outcomes in refractive cataract surgery patients who have premium IOLs.
Screen Preoperatively
Screening patients is a critical step, as both patient personality and the health status of the cornea can affect the success of the procedure.
Regarding the former, perfectionistic patients can have unrealistic expectations about their surgical outcomes, so it’s important to educate preoperative patients about the reduction of astigmatism, level of spectacle independence, and vision quality they are likely to achieve. Optimally, we want to guide the patient to make personally appropriate, educated decisions, and avoid unpleasant surprises.
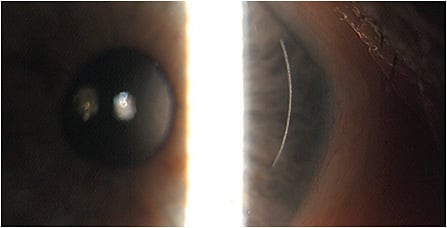
When it comes to the health status of the cornea, carefully examining patients for corneal conditions, such as active anterior basement membrane dystrophy (Figures 1 and 2), pterygia (Figure 3 and Figure 4), Salzmann’s nodules, keratoconus, and dry eye disease (DED), is crucial, as preexisting corneal disease can negatively impact postoperative results. (See “Typical Preoperative Tests Before Refractive Cataract Surgery,” below.)
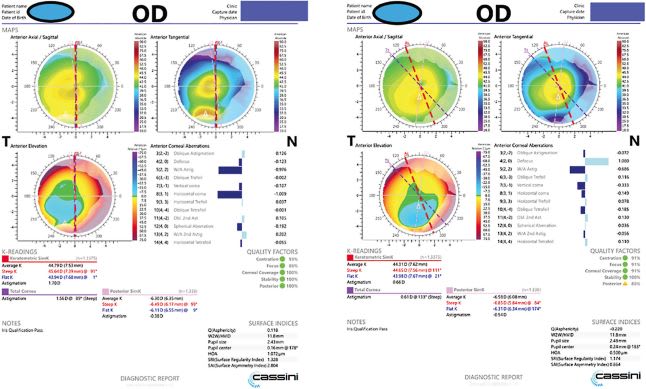
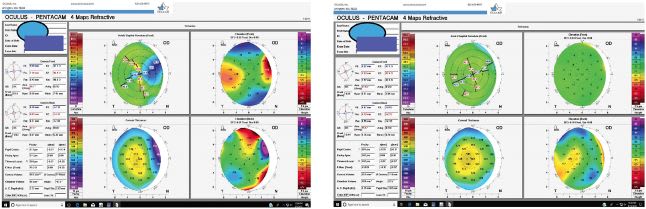
DED, in particular, interferes with preoperative measurements, recovery, and quality of vision, even if the surgery itself was successful.3,4 Of note: Gupta and colleagues reported that 80% of cataract surgery candidates had at least one sign of ocular surface dysfunction.5
Where I practice, for dry eye patients wanting refractive cataract surgery, we treat their DED for 4 weeks to 6 weeks and then repeat corneal topography. (See “Dry Eye Disease Treatments: Pharmaceutical versus Procedural,” at https://bit.ly/3jqvrT7 .) If not adequately improved, we may treat the DED longer or change our surgical plan.
We repeat corneal topography 3 months after treating anterior basement membrane dystrophy with superficial keratectomy or by removing Salzmann’s nodules. Again, if quality measurements are not obtainable, we wait longer or change our surgical plan. Patients should be counseled to accept that the change in surgical plan could be a monofocal lens instead of a multifocal, or even no refractive surgery at all if the corneal surface is not healthy enough.
Finally, we perform macular OCT on all candidates for refractive cataract surgery to rule out retinal conditions, such as vitreomacular traction.
TYPICAL PREOPERATIVE TESTS BEFORE REFRACTIVE CATARACT SURGERY
- Corneal topography with or without tear film qualifier
- Corneal wavefront mapping
- Corneal epithelial mapping
- Staining with fluorescein, lissamine green, and/or rose bengal
- Tear osmolarity
- MMP-9 testing
- OCT of the macula
- Optical biometry
Validate Measurements
Surgeons should perform multiple corneal topography measurements at different times, with, preferably, several different corneal topography devices to evaluate measurement consistency. Where I practice, we examine the patterns of the axis of astigmatism and magnitude among 3 devices on 2 different days.
To ensure accuracy, we ask patients to begin using artificial tears q.i.d. and warm compresses with eyelid hygiene b.i.d. 3 weeks before they arrive for their first preoperative examination and until the day of their surgery.
We obtain measurements when the patient is initially evaluated, and repeat them when the patient returns for their biometry and A-scan measurements. If measurements on 3 instruments show the axis at 175, 178, and 171, for example, we can easily determine the axis of treatment. However, if measurements are 178, 158, and 142, we know we will not be able to treat this patient effectively.
Posterior corneal astigmatism is an important consideration when measuring the magnitude of astigmatism.6 We use devices, such as the Cassini topographer, that can directly measure total corneal astigmatism, in addition to looking at the anterior topography measurements with theoretical calculators for total astigmatism magnitude.
Next, we determine whether the total corneal astigmatism measurements match what we would expect when we apply equations, such as the Barrett, to the anterior numbers to estimate the total corneal astigmatism. When we have consistency among all these, we know we can treat the patient confidently.
Refine Nomograms
I presented a study at the 2021 annual meeting of the American Academy of Ophthalmology demonstrating that, in using an optimized nomogram, arcuate incisions correcting 0.25 D to 0.5 D of cylinder produced a 1-line increase in uncorrected visual acuity.7
I customized my nomogram for arcuate incisions with a femtosecond laser device by beginning with the Nichamin-Woodcock nomogram and examining the results of 30 to 40 eyes2 (Figure 5). I continued to refine my settings to achieve the desired results by making specific changes within the Lensar software package. For example, I moved my radius of treatment from 4.5 mm to 4.3 mm and increased my incision depth from 85% to 90%.
Additionally, I always pair my incisions even if they are against the rule, moving my clear corneal incision one or two clock hours to the right or left of the temporal AI.
Surgeons should evaluate their own results with their laser, ensuring consistency. Everyone’s laser responds and functions uniquely due to the operative environment temperature and humidity. Subsequently, evaluating results as an ongoing practice enables continuous quality improvements and guides surgeons in refining their device settings.
I track preoperative measurements, treatment parameters, and postoperative results on a spreadsheet. There are also commercial software packages that can do the same. The important point is to do the work and track the results. We can then circle back and adjust our treatment parameters to improve future results, closing the loop on continuous quality improvement.
When considering astigmatic incisions, we should remember the hard lessons learned with RK. Large radial incisions close to the visual axis cause corneal instability. However, astigmatic incisions that are made in the arcuate fashion, if they are not made too close to the visual axis and are not too long in cord length, are very safe and very predictable.8 My current limit for incisional cylinder treatment is 1.5 D with-the-rule and 1.0 D against-the-rule astigmatism, and they are always pared.
Beyond the Surgical Suite
Patient care does not end in the operating room. Surgeons and staff must emphasize to the patient the importance of following our post-surgery recommendations for cystoid macular edema prophylaxis and DED, among other comorbidities. Also, surgeons should be alert for early posterior capsular opacification. It can interfere with postoperative refractive results. (In such cases, patients may blame the surgeon for unsatisfactory vision when they simply need a capsulotomy.) CP
References:
- Alkatan 1. Schallhorn SC, Hettinger KA, Pelouskova M, et al. Effect of residual astigmatism on uncorrected visual acuity and patient satisfaction in pseudophakic patients. J Cataract Refract Surg. 2021;47(8):991-998.
- Visco DM, Bedi R, Packer M. Femtosecond laser-assisted arcuate keratotomy at the time of cataract surgery for the management of preexisting astigmatism. J Cataract Refract Surg. 2019;45(12):1762-1769. Erratum in: J Cataract Refract Surg. 2020;46(4):658.
- Epitropoulos AT, Matossian C, Berdy GJ, Malhotra RP, Potvin R. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41(8):1672-1677.
- Szakáts I, Sebestyén M, Tóth É, Purebl G. Dry eye symptoms, patient-reported visual functioning, and health anxiety influencing patient satisfaction after cataract surgery. Curr Eye Res. 2017;42(6):832-836.
- Gupta PK, Drinkwater OJ, VanDusen KW, Brissette AR, Starr CE. Prevalence of ocular surface dysfunction in patients presenting for cataract surgery evaluation. J Cataract Refract Surg. 2018;44(9):1090-1096.
- Koch DD, Ali SF, Weikert MP, Shirayama M, Jenkins R, Wang L. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg. 2012;38(12):2080-2087.
- Visco DM. Femtosecond laser image guided corneal arcuate incisions for managing mild keratometric astigmatism in cataract surgery. Paper presented at: Annual meeting of the American Academy of Ophthalmology; New Orleans, LA; November 12-15, 2021.
- Cowden JW. Radial keratotomy. A retrospective study of cases observed at the Kresge Eye Institute for six months. Arch Ophthalmol. 1982;100(4):578-80.









