After an insult to the corneal epithelium, the regeneration of the corneal surface and the maintenance of its transparency is dependent on limbal stem cells (LSCs). (See “The Limbus: An Overview,” below.) Under normal conditions, these cells divide along the limbus and migrate centripetally to close any central corneal defect.1,2 Partial (sectoral) or total, unilateral, or bilateral limbal stem cell deficiency (LSCD) leads to defective corneal healing, resulting in scarring, conjunctivalization of the cornea, opacification, neovascularization, recurrent epithelial erosions, and, therefore, even corneal blindness.3,4 What’s more, in patients who have comorbid severe dry eye disease (DED), ocular surface keratinization may also occur.5
Given the aftermath of LSCD, it is crucial that eye care providers diagnose and treat it promptly. Here, we discuss how to accomplish both.
Diagnosis
The destruction or dysfunction of the LSCs may occur as a primary condition or may be secondary to various etiologies, such as aniridia or Turner syndrome, among others.
Diagnosis is mostly clinical, based on slit lamp examination with fluorescein staining. However, this method is highly subjective and shows considerable inter-observer variations.6 For these reasons, the diagnosis must be confirmed with a combination of impression cytology (IC)7 and in vivo confocal microscopy (IVCM).8 Additionally, in the last decade, high-resolution (HR)-OCT and HR-OCT-A, which are rapid, non-contact imaging techniques requiring low operator dependence, have been used successfully in the diagnosis of LSCD.9,10
Of note: Ruling out this diagnosis before performing a corneal transplant is crucial, as corneal transparency will not ensue unless the limbal niche is replaced by healthy LSCs via LSC transplantation.1,4,11
THE LIMBUS: AN OVERVIEW
The limbus constitutes the transition zone between the opaque sclera and the transparent cornea.58 Limbal stem cells (LSCs) are a quiescent progenitor cell population with a high proliferative capacity that reside in the basal epithelial layer of the limbus.59 These cells are embedded in a specialized microenvironment that contains cellular and non-cellular elements called the “limbal niche,” which is organized in an anatomical pattern known as the palisades of Vogt.60 The palisades of Vogt form crypts in the epithelium of the limbus, allowing for close contact between LSCs and other supportive cells: keratocytes, melanocytes, Langerhans cells, and mesenchymal stem cells, that, along with the basement membrane, nerves, and vasculature, provide the required nutrients and growth factors to promote adequate LSC proliferation and differentiation.61-66
Treatment
The LSCD treatment approach is decided based on the etiology and the clinical presentation of the condition: the extent of involvement of the limbus (partial or total), unilateral or bilateral disease, with a wet or dry ocular surface versus a keratinized ocular surface.1,4
Independently of the desired approach, the optimization of the ocular surface is imperative and should, therefore, always be the first step in the management of LSCD. Comorbid conditions, both systemic (i.e. autoimmune diseases like Sjögren’s syndrome) or localized (e.g., DED, anatomical defects, tumors, infections, use of contact lenses) must be addressed and controlled before attempting any therapeutic option, whether medical or surgical, to reduce the chance of treatment failure due to inflammation on the ocular surface.5
The reason to chose one of the presented options over another is dependent on the specific case and the ophthalmologist’s preference. There are no algorithms or “perfect techniques” for each condition.
Interventions for partial or total LSCD:
- Artificial tears/autologous serum tears. In partial LSCD, improving the ocular surface provides a better environment for the viable LSCs to survive, and in total LSCD, it improves the outcomes of limbal graft transplants. This can be achieved by maintaining ocular surface wetness by using artificial tears (ATs)5 or blood-derived products (i.e., autologous serum tears, plasma rich in growth factors), which provide growth factors and nutrients that regular ATs do not.11-17 Sharma et al demonstrated that the use of umbilical cord serum therapy after ocular chemical burns decreased the extent of limbal ischemia by 73%, as compared to 44% seen with the use of ATs, a fact attributed to the growth factors present in the serum, which stimulate the proliferation of LSCs after the insult.18
- Scleral contact lenses. These constitute another valuable option,19 and if ocular surface inflammation coexists, it should also be addressed with topical anti-inflammatories (e.g., cyclosporine, corticosteroids).20 Scleral contact lenses allow for the creation of a healthier ocular surface environment by providing lubrication as well as protection from mechanical issues, such as trichiasis. They also help to correct the irregular astigmatism that many of these patients have due to corneal scarring.Control of systemic autoimmune diseases should be achieved with systemic immunomodulatory therapy. This approach could be coupled with amniotic membrane transplantation (AMT).
- AMT. Tseng et al. reported that AMT promoted expansion of the remaining LSCs in patients who had partial LSCD more than 20 years previous.21 Since then, many investigators have used AMT to treat LSCD, both partial and total. However, when total, an additional source of limbal stem cells (autologous versus allogenic) is required along with the AMT.22 AMT grafts provide anti-inflammatory, antifibrotic antiangiogenic and antimicrobial properties, promoting epithelial cell migration into affected areas and preventing scar tissue from taking over the regeneration process, which makes them an ideal and first-line therapeutic option for acute limbal injuries (e.g., chemical burns, Stevens-Johnson syndrome, and infections).22
- Tenonplasty. This may be a rapid intervention to try to reestablish the limbal blood supply in patients who have a severe acute chemical injury and have severe ishemia of the limbus. A tenonplasty will allow for better blood flow to the limbus, decreasing the chances of stromal melting and making it easier for future surgical rehabilitation with a limbal stem cell transplant. It can be combined with AMT23 or with buccal mucosal autografts.24 After stabilizing the ocular surface, LSC transplantation becomes the utmost solution to restore the affected corneal tissue.1 Yet, there is a lack of standardization or a protocol for deciding a particular intervention for achieving this.25
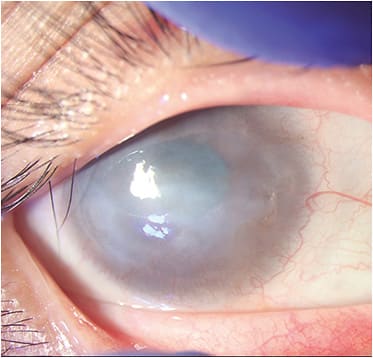
Interventions for partial, unilateral LSCD, with a wet ocular surface:
- Conjunctival/corneal epitheliectomy. This is the mechanical scraping of the epithelium from the affected corneal surface. It may be enough for treating cases of partial, unilateral LSCD that have a wet ocular surface, as LSCs from healthy limbal portions divide and migrate to cover the epithelial defect, if present.5
- Conjunctival-limbal autograft (CLAU). In this procedure, the healthy limbal tissue of the fellow eye (using the conjunctiva as the carrier for LSCs) is transplanted to the eye that has LSCD.26 The fellow donor eye must be evaluated prior to the procedure to ensure no significant ocular abnormalities, such as severe dryness or LSCD signs, are present. The recipient eye must be assessed to ensure it’s in optimal condition to avoid tissue wastage. The most feared complication is inducing LSCD in the donor eye, so a detailed informed consent about the risks to the fellow eye must be thoroughly discussed with the patient.27 AMT can be used as an adjuvant technique, if desired.28
- Autologous ex vivo cultivated limbal epithelial transplantation (Auto-CLET). Here, LSCs are harvested by a small limbal biopsy from the fellow donor eye, similar to CLAU, but instead of using conjunctival tissue as a scaffold, the cells are cultured ex vivo using other carriers (e.g., AMTs or a fibrin-based substrate).4 The main advantage of this technique is that it carries less risk of iatrogenic LSCD because it requires a smaller size of limbal biopsy from the donor eye. Technologic requirements and a high cost are associated with cell culturing.29
- Autologous simple limbal epithelial transplantation (Auto-SLET). In this procedure, a small donor limbal graft from the fellow donor eye is harvested, but instead of being expanded ex vivo, it is cut into small pieces and expanded in vivo in the affected eye, with the aid of an amniotic membrane and fibrin glue.30In coauthor Dr. Amescua’s experience, SLET works very well in patients who have purely corneal LSCD that has an overall healthy conjunctiva. But if there is conjunctival fibrosis/symblepharon, SLET conjunctival autograft from the contralateral eye can be done or the CLAU technique can be used.
These techniques do not require immunosuppression because the transplanted cells belong to the patient.

Interventions for bilateral LSCD, with a wet ocular surface:
- Keratolimbal allograft (KLAL). Here, cadaveric limbal and corneal tissue are transplanted, making this procedure convenient for severe bilateral LSCD and in cases of predominant limbal infection that has mild conjunctival involvement.31 This procedure requires systemic immunosuppression to prevent graft rejection or failure. Graft survival rate is known to decrease dramatically over 2 years,32 and patients can present with adverse effects, such as elevated liver function markers and hyperglycemia, related to long-term immunosuppression.33,34
- Living related conjunctival-limbal allograft (Lr-CLAL). As with CLAU, conjunctival and limbal tissue are transplanted in this procedure, but because the disease is bilateral, these tissues would come from a living relative (e.g., parent or sibling).35 Systemic immunosuppression is required to prevent rejection or failure of the allograft.33,34 As in KLAL, graft survival rate declines substantially over 2 years.36
- Allo-CLET and Allo-SLET. These are the same procedures as Auto-CLET and Auto-SLET, but grafts are obtained from living relatives or cadaveric donors and, therefore, immunosuppression is required.4,37
- Cultivated oral mucosal epithelial transplantation (COMET). In this procedure, oral mucosa of the LSCD patient is harvested and cut into explants, then cultured on an amniotic membrane for 3 weeks, so that a confluent epithelial sheet is produced. This sheet is then sutured to the limbus, and a contact lens is placed on top as a bandage.38-40 This technique does not require long-term systemic immunosuppression because the graft is autologous.
- Boston keratoprosthesis Type 1. This is an alternative to cell-based therapies in patients who cannot be immunosuppressed or who have previously failed LSC allografts.41 New-onset or progression of pre-existing glaucoma, a retroprosthetic membrane, and infectious endophthalmitis are the most common complications of this approach.42 Secondary glaucoma, infections, and retinal detachments are some of the other complication that are associated with this intervention.
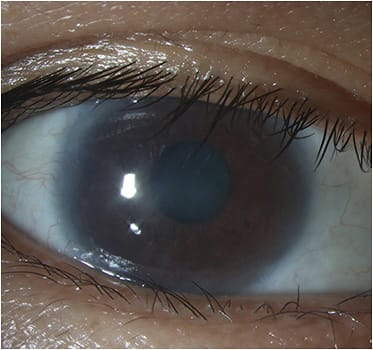
Interventions for bilateral LSCD, with a keratinized ocular surface:
- Boston keratoprosthesis Type 2. This procedure is reserved for patients who have extensive symblepharon, ankyloblepharon or a keratinized ocular surface (e.g., end-stage Stevens-Johnson syndrome or burnt-out mucous membrane pemphigoid).43 Stripping of the bulbar and palpebral conjunctivae, shaping of the eyelids around the anterior optic, and tarsorraphy are required. Visual prognosis and long-term retention rates remain guarded.43-45
- Modified osteo-odonto-keratoprosthesis (MOOKP). This is reserved for bilaterally blind patients who have end-stage ocular surface disease where simpler options have been ruled out. It consists of a synthetic optical cylinder, supported by an osteodental lamina. MOOKP provides visual rehabilitation and a long-term anatomically stable prosthesis.46,47 Complications, such as secondary glaucoma, are associated with this procedure.
Interventions for chronic sequelae of LSCD:
- Mucous membrane grafts (MMG). MMG can be used to reconstruct the fornix and eyelid margins to treat cicatricial residual disease.48 To avoid recurrent microtrauma from keratinized eyelids or fibrotic tarsal conjunctiva, strips of keratinized or fibrotic tissue are excised and replaced with oral MMG, which is then sutured or glued with fibrin glue.49-52
- Minor salivary gland trans-plantation (MSGT). This technique transplants clusters of salivary secretory cells to the ocular surface. These cells continuously produce a fluid similar to tears.53,54
Future Treatments?
Umbilical cord, bone marrow, fat, dental pulp, hair follicle, and pluripotent stem cells or mesenchymal stem cells (MSCs) have shown encouraging results in ocular surface injury models.4,55 MSCs have shown anti-neovascularization, anti-inflammatory effects, and healing properties in animal models of chemical injury.56 Pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium have been used to generate corneal epithelial cells.57 There is insufficient data regarding successful use of these cells in humans and their long-term efficacy. This limits their capacity to be used as an actual treatment approach for corneal regeneration in LSCD at this time, but, perhaps, they could be treatments down the road. CP
References:
1. Atallah MR, Palioura S, Perez VL, Amescua G. Limbal stem cell transplantation: current perspectives. Clin Ophthalmol. 2016;10:593-602.
2. Dua HS, Forrester JV. The corneoscleral limbus in human corneal epithelial wound healing. Am J Ophthalmol. 1990;110(6):646-656.
3. Bonnet C, Roberts JS, Deng SX. Limbal stem cell diseases. Exp Eye Res. 2021;205:108437.
4. Cabral-Macias J, Martinez JD, Naranjo A, Amescua G. Update on the surgical reconstruction of ocular surface in eyes with limbal stem cell deficiency. Curr Ophthalmol Rep. 2018;6:256-265.
5. Sejpal K, Bakhtiari P, Deng SX. Presentation, diagnosis and management of limbal stem cell deficiency. Middle East Afr J Ophthalmol. 2013;20(1):5-10.
6. Jawaheer L, Anijeet D, Ramaesh K. Diagnostic criteria for limbal stem cell deficiency-a systematic literature review. Surv Ophthalmol. 2017;62(4):522-532.
7. Donisi PM, Rama P, Fasolo A, Ponzin D. Analysis of limbal stem cell deficiency by corneal impression cytology. Cornea. 2003;22(6):533-538.
8. Deng SX, Sejpal KD, Tang Q, Aldave AJ, Lee OL, Yu F. Characterization of limbal stem cell deficiency by in vivo laser scanning confocal microscopy: a microstructural approach. Arch Ophthalmol. 2012;130(4):440-445.
9. Le Q, Xu J, Deng SX. The diagnosis of limbal stem cell deficiency. Ocul Surf. 2018;16(1):58-69.
10. Varma S, Shanbhag SS, Donthineni PR, Mishra DK, Singh V, Basu S. High-resolution optical coherence tomography angiography characteristics of limbal stem cell deficiency. Diagnostics (Basel). 2021;11(6):1130.
11. Baradaran-Rafii A, Eslani M, Haq Z, Shirzadeh E, Huvard MJ, Djalilian AR. Current and upcoming therapies for ocular surface chemical injuries. Ocul Surf. 2017;15(1):48-64.
12. Tovar AA, White IA, Sabater AL. Use of acellular umbilical cord-derived tissues in corneal and ocular surface diseases. Medicines (Basel). 2021;8(2):12.
13. Giannaccare G, Versura P, Buzzi M, Primavera L, Pellegrini M, Campos EC. Blood derived eye drops for the treatment of cornea and ocular surface diseases. Transfus Apher Sci. 2017;56(4):595-604.
14. Buzzi M, Versura P, Grigolo B, et al. Comparison of growth factor and interleukin content of adult peripheral blood and cord blood serum eye drops for cornea and ocular surface diseases. Transfus Apher Sci. 2018;57(4):549-555.
15. Tovar ASA. Autologous blood products: when, where, and how? Curr Ophthalmol Rep. 2021;9(2):48-56.
16. Lopez-Plandolit S, Morales MC, Freire V, Grau AE, Duran JA. Efficacy of plasma rich in growth factors for the treatment of dry eye. Cornea. 2011;30(12):1312-1317.
17. Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000;19(1):113-129.
18. Sharma N, Goel M, Velpandian T, Titiyal JS, Tandon R, Vajpayee RB. Evaluation of umbilical cord serum therapy in acute ocular chemical burns. Invest Ophthalmol Vis Sci. 2011;52(2):1087-1092.
19. Schornack MM. Limbal stem cell disease: management with scleral lenses. Clin Exp Optom. 2011;94(6):592-594.
20. Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71-82.
21. Tseng SC, Prabhasawat P, Barton K, Gray T, Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116(4):431-441.
22. Sabater AL, Perez VL. Amniotic membrane use for management of corneal limbal stem cell deficiency. Curr Opin Ophthalmol. 2017;28(4):363-369.
23. Casas VE, Kheirkhah A, Blanco G, Tseng SC. Surgical approach for scleral ischemia and melt. Cornea. 2008;27(2):196-201.
24. Wang S, Tian Y, Zhu H, Cheng Y, Zheng X, Wu J. Tenonplasty combined with free oral buccal mucosa autografts for repair of sclerocorneal melt caused by chemical burns. Cornea. 2015;34(10):1240-1244.
25. Ganger A, Singh A, Kalaivani M, et al. Outcomes of surgical interventions for the treatment of limbal stem cell deficiency. Indian J Med Res. 2021;154(1):51-61.
26. Krysik K, Miklaszewski P, Dobrowolski D, Lyssek-Boron A, Grabarek BO, Wylegala E. Ocular surface preparation before keratoprosthesis implantation. Ophthalmol Ther. 2022;11(1):249-259.
27. Daya SM. Conjunctival-limbal autograft. Curr Opin Ophthalmol. 2017;28(4):370-376.
28. Barreiro TP, Santos MS, Vieira AC, de Nadai Barros J, Hazarbassanov RM, Gomes JA. Comparative study of conjunctival limbal transplantation not associated with the use of amniotic membrane transplantation for treatment of total limbal deficiency secondary to chemical injury. Cornea. 2014;33(7):716-720.
29. Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349(9057):990-993.
30. Sangwan VS, Basu S, MacNeil S, Balasubramanian D. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96(7):931-934.
31. Cheung AY, Holland EJ. Keratolimbal allograft. Curr Opin Ophthalmol. 2017;28(4):377-381.
32. Ilari L, Daya SM. Long-term outcomes of keratolimbal allograft for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109(7):1278-1284.
33. Krakauer M, Welder JD, Pandya HK, Nassiri N, Djalilian AR. Adverse effects of systemic immunosuppression in keratolimbal allograft. J Ophthalmol. 2012;2012:576712.
34. Holland EJ, Mogilishetty G, Skeens HM, et al Systemic immunosuppression in ocular surface stem cell transplantation: results of a 10-year experience. Cornea. 2012;31(6):655-661.
35. Kwitko S, Marinho D, Barcaro S, et al Allograft conjunctival transplantation for bilateral ocular surface disorders. Ophthalmology. 1995;102(7):1020-1025.
36. Santos MS, Gomes JA, Hofling-Lima AL, Rizzo LV, Romano AC, Belfort R Jr. Survival analysis of conjunctival limbal grafts and amniotic membrane transplantation in eyes with total limbal stem cell deficiency. Am J Ophthalmol. 2005;140(2):223-230.
37. Movahedan A, Cheung AY, Eslani M, Mogilishetty G, Govil A, Holland EJ. Long-term outcomes of ocular surface stem cell allograft transplantation. Am J Ophthalmol. 2017;184:97-107.
38. Nishida K, Yamato M, Hayashida Y, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351(12):1187-1196.
39. Burillon C, Huot L, Justin V, et al. Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Invest Ophthalmol Vis Sci. 2012;53(3):1325-1331. Epub 2011/11/09. doi: 10.1167/iovs.11-7744. PubMed PMID: 22064987.
40. Nakamura T, Inatomi T, Sotozono C, Amemiya T, Kanamura N, Kinoshita S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol. 2004;88(10):1280-1204.
41. Sejpal K, Yu F, Aldave AJ. The Boston keratoprosthesis in the management of corneal limbal stem cell deficiency. Cornea. 2011;30(11):1187-1194.
42. Shanbhag SS, Senthil S, Mohamed A, Basu S. Outcomes of the Boston type 1 and the Aurolab keratoprosthesis in eyes with limbal stem cell deficiency. Br J Ophthalmol. 2021;105(4):473-478.
43. Pujari S, Siddique SS, Dohlman CH, Chodosh J. The Boston keratoprosthesis type II: the Massachusetts Eye and Ear Infirmary experience. Cornea. 2011;30(12):1298-1303.
44. Saeed HN, Shanbhag S, Chodosh J. The Boston keratoprosthesis. Curr Opin Ophthalmol. 2017;28(4):390-396.
45. Gomaa A, Comyn O, Liu C. Keratoprostheses in clinical practice — a review. Clin Exp Ophthalmol. 2010;38(2):211-224.
46. Reddy SC, Tajunisah I, Tan DT. Osteo-odonto keratoprosthesis in Stevens-Johnson syndrome: a case report. Int J Ophthalmol. 2011;4(2):212-215..
47. Falcinelli G, Falsini B, Taloni M, Colliardo P, Falcinelli G. Modified osteo-odonto-keratoprosthesis for treatment of corneal blindness: long-term anatomical and functional outcomes in 181 cases. Arch Ophthalmol. 2005;123(10):1319-1329.
48. Fu Y, Liu J, Tseng SC. Oral mucosal graft to correct lid margin pathologic features in cicatricial ocular surface diseases. Am J Ophthalmol. 2011;152(4):600-608.
49. Eslani M, Baradaran-Rafii A, Ahmad S. Cultivated limbal and oral mucosal epithelial transplantation. Semin Ophthalmol. 2012;27(3-4):80-93.
50. Takeda K, Nakamura T, Inatomi T, Sotozono C, Watanabe A, Kinoshita S. Ocular surface reconstruction using the combination of autologous cultivated oral mucosal epithelial transplantation and eyelid surgery for severe ocular surface disease. Am J Ophthalmol. 2011;152(2):195-201.
51. Iyer G, Pillai VS, Srinivasan B, Guruswami S, Padmanabhan P. Mucous membrane grafting for lid margin keratinization in Stevens-Johnson syndrome: results. Cornea. 2010;29(2):146-151.
52. Kheirkhah A, Ghaffari R, Kaghazkanani R, Hashemi H, Behrouz MJ, Raju VK. A combined approach of amniotic membrane and oral mucosa transplantation for fornix reconstruction in severe symblepharon. Cornea. 2013;32(2):155-160.
53. Sant' Anna AE, Hazarbassanov RM, de Freitas D, Gomes JA. Minor salivary glands and labial mucous membrane graft in the treatment of severe symblepharon and dry eye in patients with Stevens-Johnson syndrome. Br J Ophthalmol. 2012;96(2):234-239.
54. Marinho DR, Burmann TG, Kwitko S. Labial salivary gland transplantation for severe dry eye due to chemical burns and Stevens-Johnson syndrome. Ophthalmic Plast Reconstr Surg. 2010;26(3):182-184.
55. Nurkovic JS, Vojinovic R, Dolicanin Z. Corneal stem cells as a source of regenerative cell-based therapy. Stem Cells Int. 2020;2020:8813447.
56. Javorkova E, Trosan P, Zajicova A, Krulova M, Hajkova M, Holan V. Modulation of the early inflammatory microenvironment in the alkali-burned eye by systemically administered interferon-gamma-treated mesenchymal stromal cells. Stem Cells Dev. 2014;23(20):2490-2500.
57. Hayashi R, Ishikawa Y, Ito M, et al. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PLoS One. 2012;7(9):e45435.
58. Van Buskirk EM. The anatomy of the limbus. Eye (Lond). 1989;3(Pt 2):101-108.
59. Gonzalez G, Sasamoto Y, Ksander BR, Frank MH, Frank NY. Limbal stem cells: identity, developmental origin, and therapeutic potential. Wiley Interdiscip Rev Dev Biol. 2018;7(2):10.1002/wdev.303.
60. Amin S, Jalilian E, Katz E, et al. The limbal niche and regenerative strategies. Vision (Basel). 2021;5(4):43.
61. Yazdanpanah G, Haq Z, Kang K, Jabbehdari S, Rosenblatt ML, Djalilian AR. Strategies for reconstructing the limbal stem cell niche. Ocul Surf. 2019;17(2):230-240.
62. Higa K, Shimmura S, Miyashita H, Shimazaki J, Tsubota K. Melanocytes in the corneal limbus interact with K19-positive basal epithelial cells. Exp Eye Res. 2005;81(2):218-223.
63. Polisetti N, Zenkel M, Menzel-Severing J, Kruse FE, Schlotzer-Schrehardt U. Cell adhesion molecules and stem cell-niche-interactions in the limbal stem cell niche. Stem Cells. 2016;34(1):203-219.
64. Katikireddy KR, Jurkunas UV. Limbal stromal tissue specific stem cells and their differentiation potential to corneal epithelial cells. Methods Mol Biol. 2016;1341:437-444.
65. Chidambaranathan GP, Mathews S, Panigrahi AK, Mascarenhas J, Prajna NV, Muthukkaruppan V. In vivo confocal microscopic analysis of limbal stroma in patients with limbal stem cell deficiency. Cornea. 2015;34(11):1478-1486.
66. Huang M, Wang B, Wan P, et al. Roles of limbal microvascular net and limbal stroma in regulating maintenance of limbal epithelial stem cells. Cell Tissue Res. 2015;359(2):547-563.










