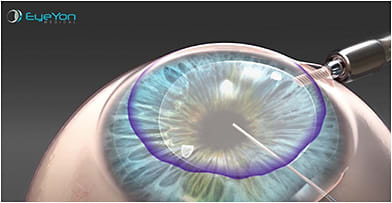
World’s Thinnest 3D-Printed Cornea Successfully Transplanted
What a difference a year makes! In the “News” section of the July 2021 issue of Corneal Physician, an article appeared on the creation of a ready-to-use biocompatible endothelial graft called EndoArt (EyeYon) to eliminate the requirement of donor tissue for corneal edema treatment. (See bit.ly/July2021NewsCP .) At that time, EndoArt was undergoing clinical trials in Asia, Europe, and Israel.
A few months ago, a 50 μm EndoArt cornea, which was 3D printed from a sterile biocompatible acrylic, was successfully transplanted into a 71-year-old man who suffered from corneal edema and failed a previous donated tissue transplant, reported The Jerusalem Post. The procedure occurred at Israel’s Shaare Zedek Medical Center.
“This is another step toward a future in which the dependence on the availability of human tissue for the purpose of performing corneal transplants in patients who need it will be reduced,” surgeon Liron Berkovich explained to the Israeli newspaper.
Refractive Surgery Pioneer Passes
 James Joseph Salz, MD, a trailblazer in refractive surgery, recently died at age 82, reported The Los Angeles Times.
James Joseph Salz, MD, a trailblazer in refractive surgery, recently died at age 82, reported The Los Angeles Times.
Dr. Salz is a member of an exclusive group of U.S. surgeons who have performed all refractive surgery types, including keratomileusis and PRK, according to his bio for his practice Laser Vision Medical Associates. Additional accomplishments include the role of principle investigator on several FDA clinical trials for such companies as Alcon, STAAR Surgical, and VISX, Inc; numerous book chapters; and more than 80 articles on cataract, refractive, and lens implant surgery and contact lenses.
Also, Dr. Salz was co-editor of the book Refractive Corneal Surgery; editor-in-chief of the book Corneal Laser Surgery; and founding editor of the Journal of Refractive Surgery.
The prolific ophthalmic surgeon was past president of the International Society of Refractive Keratoplasty and the International Society of Refractive Surgeons; chairman of the American Academy of Ophthalmology (AAO) Refractive Surgery Interest Group; and past executive committee chairman of the International Society of Refractive Surgeons. He received the International Society of Refractive Surgery Barraquer Award for his contributions to the refractive surgery field in 1994, the AAO’s Senior Honor Award in 1997, and the AAO’s Lifetime Achievement Award in 2013.
Dr. Salz, an avid sailor, skier, and fan of Duke University basketball, is survived by his wife Judith; his children, James, Mark (Monica), Heather (Scott), and Elisabeth (Kevin); his grandchildren, Emily, Riley, Brody, Matthew, Ella, Sam, Emmett, Grace, Lucy, and Hazel; his sister, Audrey; and his nephew, David.

Cataract Surgery Goes Swimmingly for Fish
 Dill Prickle, a long-spine porcupine pufferfish — think “Bloat” from Finding Nemo — who swims at the Columbus Zoo and Aquarium, in Powell, Ohio, recently underwent successful cataract surgery for lens luxation. Katie Seeley, staff veterinarian, said this condition, if untreated, can cause glaucoma and potentially lead to eye removal, according to a video posted on the Zoo’s Facebook page.
Dill Prickle, a long-spine porcupine pufferfish — think “Bloat” from Finding Nemo — who swims at the Columbus Zoo and Aquarium, in Powell, Ohio, recently underwent successful cataract surgery for lens luxation. Katie Seeley, staff veterinarian, said this condition, if untreated, can cause glaucoma and potentially lead to eye removal, according to a video posted on the Zoo’s Facebook page.
Specifically, Mr. Prickle was removed from the water, then had water containing anesthetic administered over his gills via syringe and delivered through a tube into his mouth. Terah Webb, DVM, DACVOM, a veterinary ophthalmologist, performed the surgery, which took 20 minutes.
Mr. Prickle is now back to swimming and eating crustaceans, other fish, shrimp, and squid.
To follow Mr. Prickle’s progress, visit facebook.com/columbuszoo .

.
ALSO NOTEWORTHY
- Alcon’s IQ PanOptix Trifocal IOL has been implanted more than one million times worldwide, spurring the company to create the “One Million Moments — Thank You” ad campaign that recognizes the positive impact eye care providers have made on those patients implanted with the IOL.
- Amferia AB was granted a foundational patent by the U.S. Patent and Trademark Office for its material that uses covalent binding between antimicrobial peptides and an amphiphilic hydrogel to form a solid antimicrobial hydrogel material. The material exerts an antibacterial effect upon direct contact with bacteria, without leaching or release of any antimicrobials into the environment around the material, Amferia AB says. The company says it is in the final stages of product creation for wound care applications. The patent number is US 11235021.
- Avellino Lab USA named Genya Dana, PhD, as global head of health policy for the company. Dr. Dana previously served as the head of health care at the World Economic Forum, where she oversaw an international team focused on creating multi-stakeholder health care partnerships, including initiatives in precision medicine, women’s and girls’ health, health equity, and pandemic forecasting and response.
- AXIM Biotechnologies, Inc announced it created a rapid quantitative tear test for MMP-9, an inflammatory biomarker for dry eye disease. “The availability of quantitative tear MMP-9 testing would be a significant advance in our ability to measure the degree of inflammation affecting our dry eye patients, allowing for more objective classification of their disease,” said Dr. Joseph Tauber, AXIM’s chief medical officer, in a press release. “Equally important would be the ability to measure improvement in control of inflammation that is the goal of many of our therapies for ocular surface disease, including pharmaceuticals, thermal pulsation treatments, and even light-based therapies.”
- Graybug Vision, Inc recently acquired RainBIO, which created a gene therapy for mucopolysaccharidosis type 1 (MPS1), an inherited lysosomal storage disorder that has a high prevalence of corneal clouding. The company’s adeno-associated virus vector (AAV) gene therapy, called GB-501, has received Orphan Drug Designation from the FDA and the company is preparing for phase 1/2a trials.
- Johnson & Johnson Vision was recognized as the winner of Fast Company’s 2022 World Changing Ideas Awards in the Wellness category for the company’s Acuvue Theravision with Ketotifen, a drug-eluting contact lens intended for patients suffering from ocular itch due to allergic conjunctivitis. Each of the daily disposable contact lenses contains 19 mcg of ketotifen, an antihistamine. The World Changing Ideas Awards honor products, concepts, companies, policies, and designs that are pursuing innovation for the good of society and the planet, reported the press release. For more information on the drug-eluting contact lens, visit acuvuetheravision.com .
- Kala Pharmaceuticals, Inc announced it has entered into a definitive agreement to sell its commercial portfolio and related intellectual property assets to Alcon Inc. This includes Eysuvis, FDA-approved for the short-term (up to two weeks) treatment of the signs and symptoms of dry eye disease, and Inveltys, a b.i.d. corticosteroid for the treatment of postoperative inflammation and pain following ocular surgery.
- Lumibird Medical launched the Capsulo Nd:YAG laser, which provides efficient and accurate capsulotomy and iridotomy treatments, using optics that deliver clarity of view into the anterior and posterior segments of the eye and illumination with a variable-height light tower that provides dual illumination angles of 16° and 7.5° for anterior and posterior laser applications, the company said. The laser can be paired with Quantel Medical’s Vitra 2 MultiSpot photocoagulating laser to combine panretinal photo-coagulation functionality, Lumbird said.
- Ocuphire Pharma, Inc named Jay Pepose, MD, PhD, its chief medical advisor. Dr. Pepose has served on Ocuphire’s medical advisory board since 2018 and on the board of directors since 2021. He is the founder and medical director of the Pepose Vision Institute in the St. Louis area; a professor of clinical ophthalmology and visual sciences at Washington University School of Medicine in St. Louis; and a consultant to the Centers for Disease Control and Prevention in Atlanta.
- OptiLight, from Lumenis, received the MedTech Breakthrough Award for Best New Technology Solution for Ophthalmology. There were more than 3,900 nominations from 15 countries, according to the press release.
- Orasis Pharmaceuticals appointed Teresa “Tes” Ignacio, MD, as vice president of medical affairs. Dr. Ignacio, a fellowship-trained ophthalmologist, brings over a decade of experience in research and development, clinical practice, and medical affairs, according to the press release. She trained as a cornea and refractive research fellow at the University of California Irvine, and completed her ophthalmology residency at Makati Medical Center in the Philippines. In other news, Orasis announced the completion of its NEAR-1 and NEAR-2 phase 3 clinical studies, which evaluated the efficacy and safety of CSF-1, the company’s eye drop candidate for the treatment of presbyopia.
- Salvat has successfully concluded phase 3 clinical trials of its ocular corticosteroid eye drop treatment for inflammation and pain in cataract surgery patients. The Spanish pharmaceutical company said it has begun the approval application procedures for clobetasol propionate ophthalmic nanoemulsion 0.05% with the FDA as well as with European health authorities, and hopes to launch the corticosteroid worldwide in the second half of 2023.
- SIFI S.p.A. announced that the FDA approved the company’s Orphan Drug Designation application for its investigational anti-infective polymer polihexanide for the treatment of fungal keratitis. Polihexanide had previously been designated by the FDA and the European Medicines Agency as an orphan drug appropriate for treatment of Acanthamoeba keratitis.
- STAAR Surgical received FDA approval in March 2022 for its EVO/EVO+ Visian Implantable Collamer Lens for the correction of myopia (EVO) and myopia with astigmatism (EVO+). The product offers a lens-based alternative for the correction/reduction of refractive error in patients between the ages of 21 and 45, the company said. EVO lenses are implanted within the eye’s posterior chamber directly behind the iris and in front of the natural crystalline lens. STAAR said it is training and certifying surgeons to implant EVO lenses and will begin a national marketing campaign to support the product. CP








