Patient KB presented for a cataract surgery consultation. She reported a history of prior radial keratotomy (RK) on both eyes, enjoying “good” vision for 15 years, but gradually developing visual decline in both eyes (OU).
On presentation, the patient’s exam was remarkable for 12-incision RK OU; corneal flattening secondary to RK with central corneal keratometry of 30.37/32.06 OD, and 32.47/34.31 OS; corneal spherical aberration (SA) of 3.125 μm OD and 1.911 μm OS; and corneal higher-order aberrations (HOAs) profiles of 1.53 μm OD and 2.22 μm OS. (Figure 1 and Figure 2)
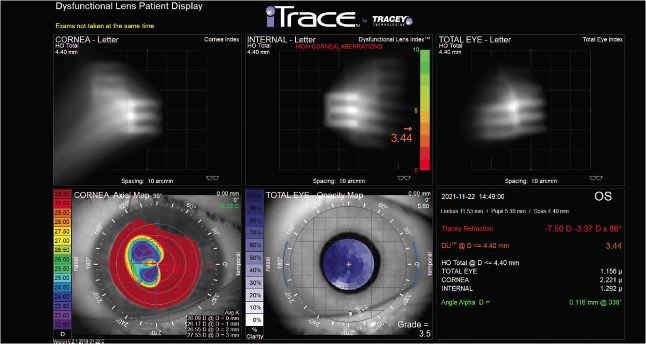
Additionally, the patient’s preoperative refractive error was -8.00 D -2.25 x 79° = 20/150 best-corrected visual acuity (BCVA) OD and -8.00 D -3.00 x 91° = 20/70-2 BCVA OS, and her cataracts were significant for 4+ nuclear opalescence and 2+ cortical changes OU.
KB said she desired spectacle independence after surgery. If you’re thinking this patient would benefit from an IOL, we are on the same wavelength. But, which IOL? To find an answer, let’s discuss the latest (within the last year, as this issue goes to press) IOL technologies available in the United States. (Future cataract patients may benefit from technologies that haven’t yet come to the U.S. market; see “In the Pipeline This Year”.)
AcuFocus IC-8 Apthera IOL
The IC-8 Apthera IOL is a non-toric, hydrophobic acrylic UV-blocking extended depth of focus IOL that has a total optic size of 6 mm internal and a total length of 12.5 mm. It features a proprietary small aperture technology, called FilterRing, which is a 1.36 mm central aperture surrounded by a 3.23 mm total diameter polyvinyldene fluoride carbon nano-particle mask that blocks the peripheral defocused rays from affecting vision. Interestingly, this small aperture design applies well to patients who have slightly hyperopic or myopic postoperative outcomes. The mask creates a pinhole effect that eliminates incidental light rays and aberrations from the peripheral cornea that may adversely effect vision. This results in functional myopia for all patients. Most patients achieve about 2.5 D of continuous depth of field. By targeting a slightly myopic result of -0.50 D to -0.75 D, this range can be extended to 3.0 D. The power range of the IC-8 lens is 10.0 D to 30.0 D.
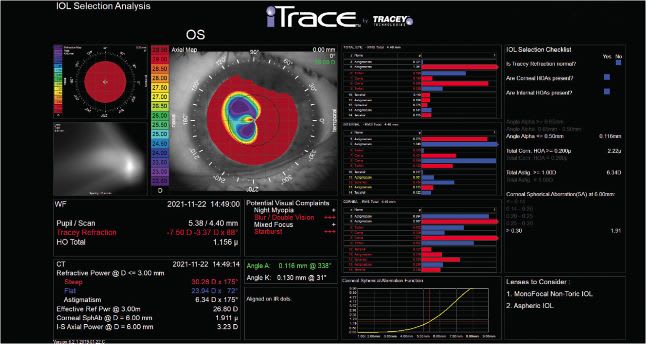
Internationally, the IC-8 Apthera IOL has demonstrated effectiveness in treating patients who have irregular corneas. Patients having undergone previous RK (Figure 3), patients with central irregularity due to keratoconus, and patients who have irregular astigmatism from prior trauma can achieve improved best-corrected vision because this lens has the ability to block peripheral defocused rays, reducing aberrations that may adversely affect vision.
The IOL is indicated for patients implanted with a monofocal IOL or monofocal toric IOL in their other eye who desire a broad range of vision without glare and halo symptoms. A caveat: We recommend avoiding using the AcuFocus IC-8 on patients who have high-angle kappa or angle alpha-D measurements, as the central aperture may not be in alignment with the patient’s line of sight.
Incidentally, prior to FDA ap-proval, one of my post-RK patients went to Germany to have an IC-8 IOL implanted in her non-dominant eye. The patient had more than 5.00 D of irregular astigmatism. Postoperatively, her manifest refraction is +0.75 D -0.75 x 92° = 20/40-2. Preoperatively her BCVA was 20/100 in the same eye. An important note with regards to this technology: While the patient is happy with her daytime vision in this eye, she does experience difficulty at night, secondary to the pinhole effects of this technology.
Alcon Clareon Family of IOLs
The Clareon family of IOLs includes Vivity, PanOptix, Monofocal, and Toric. All family members are comprised of a 1-piece hydrophobic acrylic, are available in both clear or natural, depending on surgeon preference, and are implanted via the AutonoMe delivery system, which precludes potential technician damage during the loading process.
Additionally, the standard optical biometer A-constant is 119.1 for all the Clareon IOLs, and a 6 mm optic with 13 mm modified C-loop haptics and a power range for the monofocal platform of 6.0 D to 30.0 D. The features of this family of IOLs are low levels of glare with a -0.2 μm SA treatment. In our experience, negative SA treatments commonly limit depth of field for most patients implanted with monofocal IOLs.
The Clareon material has demonstrated a significant reduction in the potential development of glistenings, which should allow patients to maintain excellent quality vision for years to come. Furthermore, a redesign of the posterior edge curve reduces the incidence of posterior capsule opacity.
Bausch + Lomb PreVue IOLs
The Bausch + Lomb (B+L) PreVue IOLs (i.e., PreVue-C and PreVue-Y) have an aberration-neutral profile, edge-to-edge uniform power, an A-constant of 118.4, 6 mm optic with a modified C-loop haptic configuration, and an overall diameter of 12.5 mm. They are individually lathe cut, 1-piece hydrophobic acrylic IOLs pre-loaded and available in either clear or with a chromophore that contains built-in UV protection.
Like many B+L IOLs that have aberration-free optics, we have found this platform functions well for patients who are post–hyperopic LASIK or post-RK, as well as those who have no prior refractive surgery with positive cornea SA up to 0.35 μm. With regards to SA effects of LASIK, hyperopic LASIK induces negative SA and, therefore, IOLs with built-in negative SA are generally not recommended for use in patients post-hyperopic LASIK. On the other hand, myopic LASIK and RK induce positive SA. If a patient has more than +0.35 μm. of corneal SA, we prefer to use negative SA IOLs to counteract this effect. Several devices are available to help surgeons identify corneal spherical aberration, including a vision assessment system that combines topography, wavefront, an automated refractor, keratometry, and pupillometry, and a device that identifies a patient’s visual axis, the center of the pupil, and the center of the limbus prior to multifocal IOL implantation.
BVI Medical IPure Family of IOLs
The BVI IPure family of monofocal IOLs is comprised of 3, 1-piece hydrophobic acrylic models: a clear 1-piece lens (B1PC), a yellow 1-piece lens (B1PY), and the clear 3-piece B3PC. The B1PC and B1PY are individually lathe cut and have an A-constant of 118.4, 6 mm optic with a modified C-loop haptic configuration and overall diameter of 12.5 mm. The B3PC is a 3-piece hydrophobic acrylic model with a similar design to the B1PC and B1PY, with a slightly larger front inject tip diameter of 1.89 mm as opposed to the diameter of 1.82 mm for the B1PC and B1PY.
IPure lenses feature a central 2.4 mm neutral SA zone with the remaining peripheral zone having -0.18 SA to aid in high-contrast vision under various lighting conditions. Coupled with -0.18 SA in the peripheral zone, patients can experience enhanced night vision contrast, as pupil size expands in low-light conditions.
Patients who have normal positive corneal SA (0.25 μm to 0.35 μm) make ideal candidates here, as the large neutral SA optical zone allows for flexibility and fewer potential issues related to centration of the IOL, along with increased depth of field.
Johnson & Johnson Tecnis Eyhance Monofocal Plus Technology
The preloaded Eyhance family of IOLs addresses the loss of depth of field and issues associated with potential decentrations in IOLs that have central negative SA treatment. The Monofocal Plus Technology IOL has a 6.0 optic, a total dimension of 13.0 mm, and the A-constant remains 119.3. The IOL’s features: an OptiBlue violet-blocking filter, proprietary shape change for a slightly extended depth of focus to reduce patients’ need of glasses in the intermediate zone of vision, and the ProTEC-frosted continuous square-edge haptic modifications to reduce the potential for IOL rotation postoperatively.
IN THE PIPELINE THIS YEAR
Rayner’s RayOne Aspheric IOL
The RayOne Aspheric IOL provides a 6.0 mm optic, 12.5 mm overall length, 105 UV cut-off at 380 nm, A-constant 118.6, and an 8.0 D to 34.0 D range. The aberration-neutral IOL features hydrophilic acrylic material with 26% water content that is glistening free; low silicone IOL adherence; uveal biocompatibility; Rayner’s anti-vault haptic (AVH) technology, which adapts to the compressive force of the capsular bag; freedom from issues potentially associated with decentration; and Rayacryl with a low index of refraction (1.46). Additionally, the RayOne Aspheric IOL has an Amon-Apple square-edge technology incorporating a 0 degree uniplanar square-edge closed-loop technology on the haptics, thereby reducing the risk of lens epithelial cell migration under the optic through the typically venerable optic haptic junction. Much like other aberration-neutral IOLs, the RayOne IOL offers enhanced depth of field by maintaining some positive spherical aberration. Ideal patients include those who have up to 0.35 μm of positive corneal SA, post–hyperopic LASIK patients, and patients who have high-angle Alpha-D or high-angle Kappa.
Patients who have monofocal femtosecond laser–assisted cataract surgery to reduce the negative effects of high positive corneal spherical aberration (greater than +0.35 μm) where there is usually a drop off in quality of vision associated with higher positive corneal SA can benefit from this IOL.
Johnson & Johnson Tecnis Symfony OptiBlue EDOF IOLs
The Symphony OptiBlue EDOF IOL family is comprised of the ZXR00V and ZXW. The ZXR00V and ZXW are UV-blocking hydrophobic acrylic IOLs with A-constants of 119.3, a 6 mm optic, and a power range of +5.0 D to 34.0 D. The features of the IOLs are extended depth of focus that provides the option for distance vision, intermediate vision, and moderate near acuity to mitigate the effects of presbyopia. Additionally, the IOLs offer night-vision contrast with minimal night-vision aberrations.
Post-LASIK patients who have low degrees of corneal HOAs can fare well in these IOLs, though we do explain to patients the potential need for low add readers to see small print. We generally implant these IOLs in patients who do most of their near work on smart phones, tablets, and computers.
A caveat: As with all diffractive optic IOLs, we suggest making sure the ocular surface has been optimized and not implanting the IOLs in patients who have a history of macular disease, so their visual outcomes can be optimized.
Johnson & Johnson Tecnis Synergy IOLs
The Tecnis Synergy IOL family is made up of the DFR00, and the DFW toric. As with the entire Tecnis platform, there is -0.28 SAs for high-contrast vision. The Tecnis Synergy IOL family and ZXW are UV-absorbing hydrophobic acrylic IOLs with A-constants of 119.3, a 6 mm optic, and a power range of +6.0 D to 34.0 D. The IOLs feature the OptiBlue violet-absorbing filter, ProTEC-frosted haptic design, a diffractive optic design on the back surface of the IOL, and a range of vision from 33 cm out to infinity.
Patients desiring reduced spectacle dependence who do not spend much time driving at night can make good candidates for these IOLs.
As with other diffractive optic IOLs, managing dry eye and ocular surface disease and avoiding implantation in patients who have prior refractive surgery or macular pathology is paramount to success with Synergy. The IOL’s eschelette design results in a narrow refractive landing zone with resulting glare and halos if the refractive target is not achieved or the patient has postoperative ocular surface issues.
RxSight Light Adjustable Lens
The RxSight Light Adjustable Lens with ActivShield is a photoreactive UV-absorbing silicone IOL that has a 10% UV cut-off at 385 nm. Other specs include a 3-piece configuration 6 mm optic, 10-degree haptic angle, 13 mm modified C loop, and A-constant 118.4 with a range of 10.0 D to 30.0 D.
The IOL allows surgeons to adjust it without returning to the OR after surgery. Most commonly, we wait 14 days to 21 days for patients to achieve refractive stability after performing cataract surgery. We typically follow the patient with serial refractions and aberrometry analysis until we have 2 equal refractions at least 2 weeks apart. Once stable, we proceed with light adjustment 4 to 7 days apart (with refractions performed in between).
For most patients, we achieve the desired refractive effect in 1 to 2 treatments. In patients who have high astigmatism, it can take up to 3 treatments to achieve the desired effect. A light delivery device is required to make these adjustments. (See “Implementing the Light Adjustable Lens in Practice,” at bit.ly/LightIOL .) It works by applying a series of light treatments to activate polymers that reshape the implanted lens using UV light. There is a maximum 2.00 D spherical equivalent adjustment that can be achieved through a combination of sphere and cylinder treatment (up to 4.00 D cyl).
Post-refractive patients can fare well with these IOLs, as we can now get LASIK-like results, achieving our target postoperative refractions most of the time. Additionally, patients who want the broadest range of vision without spectacles can achieve success, as the lens allows the surgeon to optimize a patient’s visual outcomes based on the patient’s preferences and lifestyle requirements. A caveat: To successfully perform postop light adjustments, the patient’s pupil must dilate to a minimum of 6 mm, but preferably 7 mm, for light to be applied uniformly to the lens. Patients must also be made aware that it can take 2 to 3 light treatments to achieve their vision goals, followed by 1 or 2 lock-in treatments to fix the lens permanently.
That Patient
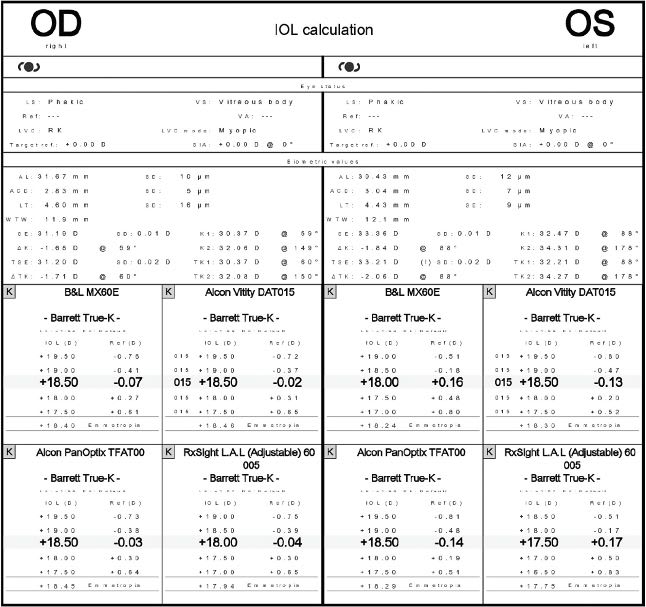
With all the knowledge above, and the lack of availability of a small aperture IOL at the time of KB’s surgery, we chose to perform surgery using the RxSight Lens with ActivShield. Because the patient’s average keratometry was below 34.0 D from prior RK, despite the apparent significant myopic shift from having dense nuclear changes, we knew there would be a potential for a progressive hyperopic shift over time. With that in mind, we targeted -0.50 D to -0.75 D spherical equivalent postoperatively in each eye. To compensate for some of the patient’s high corneal spherical aberration, the initial lens implant target was +0.30 with a plan for central steepening of the IOLs to induce additional negative spherical aberration.
We waited 8 weeks for the patient to achieve refractive stability, then proceeded with light adjustments weekly until we achieved our desired outcome.
We are happy to report that at KB’s most recent exam, her uncorrected visual acuity is 20/25 OD and 20/30 OS. Also, manifest refraction is -0.50 D sph OD and -0.75 D sph OU at distance and J5 at near without correction. CP










