Keratorefractive procedures, such as LASIK, SMILE, and PRK, often provide a safe and effective path to optimized vision for many myopic patients. However, patients who have severe ocular surface disease, high refractive error, corneal dystrophy or degeneration, or certain systemic conditions, such as collagen vascular diseases, may not be ideal candidates for keratorefractive surgery. Concerns include worsening ocular surface disease, significant tissue ablation/removal increasing aberrations and ectasia risk, delayed healing, and excessive inflammation. Fortunately, many of these patients may be able to achieve excellent vision with phakic intraocular lens implants (pIOLs).
Multiple pIOLs exist, each with distinct candidacy guidelines. In general, pIOL candidates are between the ages of 21 and 45, have a stable refractive error, and have an anterior chamber depth (ACD) and endothelial cell density (ECD) that meet defined thresholds.
Possible contraindications include small anterior chamber depth (<3.0 mm for EVO Visian ICL; <3.2 mm for Artisan/Verisyse), low ECD, or glaucoma. Each pIOL has a unique side-effect profile, but a few shared possible complications include elevated IOP, endothelial cell loss, and cataract progression. Recent improvements in lens location/fixation, materials, and architecture have made pIOLs significantly safer, but postoperative attention still needs to be paid to endothelial cells, IOP, and crystalline lens changes.
Here, we discuss the features and candidacy of the available pIOLs.
Anterior Chamber (iris-fixated)
Iris-fixation (iris claw) was introduced by Dr. Jan Worst in 1978 for aphakic correction.1 In 1986, the iris claw was adapted by Dr. Worst, Dr. Paul Fechner, and Dr. Gerrit van der Heijde for fixating a pIOL. The original design had a biconcave optic and was associated with significant rates of endothelial cell loss (13.4%).2
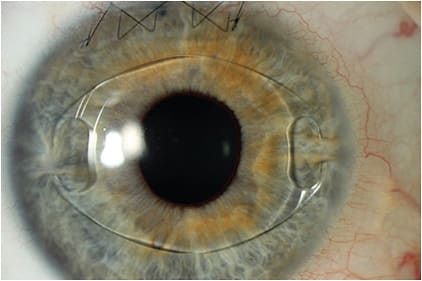
Today, the only anterior chamber iris-fixated pIOL that is available in the United States is the Artisan (Ophtec), also sold under the name Verisyse. Available in Europe since 1997, and FDA approved in 2004, this pIOL features a convex-concave optic to maintain a safe distance from the endothelium, with a vault that protects the crystalline lens and facilitates aqueous flow freely between chambers. It is made of polymethyl methacrylate (PMMA), and is inserted through a large clear corneal incision (5.5 mm to 6.2 mm).
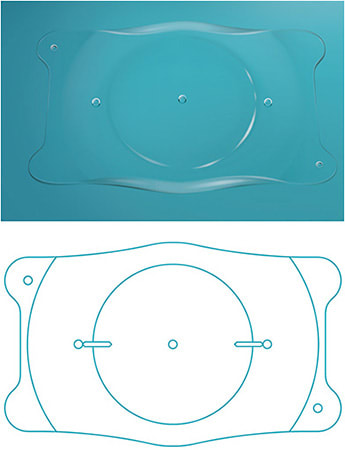
The Artisan comes in both a 5.0 mm and a 6.0 mm optic, and is secured to the mid-peripheral iris stroma by the PMMA haptics (claws). It is approved for the correction of myopia ranging from -5 D to -20 D, with ≤2.5 D of astigmatism. The Artisan lens requires an anterior chamber depth of ≥3.2 mm.
Posterior Chamber
The first posterior chamber phakic IOL was inserted by Dr. Svyatoslav N. Fyodorov in 1986—the same year as the first iris claw pIOL. Dr. Fyodorov’s pIOL inspired many other posterior chamber lenses, including the PRL from Ciba Vision and the Visian Implantable Contact/Collamer Lens (ICL) from Staar Surgical.
The original Visian ICL was approved by the Food and Drug Administration in 2005, and made of a biocompatible hydroxyethyl methacrylate (HEMA) and porcine collagen-containing polymer. The ICL had a soft, hydrophilic material with UV-absorbing attributes. The pliability facilitated injection through a 3.0 mm incision. Preoperatively, a laser peripheral iridotomy (LPI) was performed to prevent pupillary block. Earlier models have been supplanted by two posterior chamber pIOLs that are currently available in the United States:
• Visian Toric ICL (Staar Surgical). In 2018, the FDA approved the Visian Toric ICL for the correction of myopic astigmatism with spherical equivalent ranging from -3.0 D to -15.0 D, with correction of cylinder ranging from 1.0 D to 4.0 D.3
Although uncommon, spontaneous postoperative toric ICL rotations may require ICL rotation or laser vision correction enhancement. Accurate preoperative measurements and ICL size selection can mitigate this risk.
• EVO and EVO+ ICLs. FDA-approved in 2022, the EVO ICL (EVO ICL for myopia, EVO Toric ICL for myopia/astigmatism) features a 0.36 mm central port (KS-Aquaport), which permits aqueous flow through the ICL, and eliminates the need for laser iridotomy. The EVO lens has an optic diameter ranging from 4.9 mm to 5.8 mm, depending on the dioptric power.
The EVO ICL has been shown to have equal visual performance (aberration-induction profile and contrast sensitivity) versus previous implantable collamer lens models that didn’t have the central port.4 Also, the central port can reduce the rate of cataract formation.
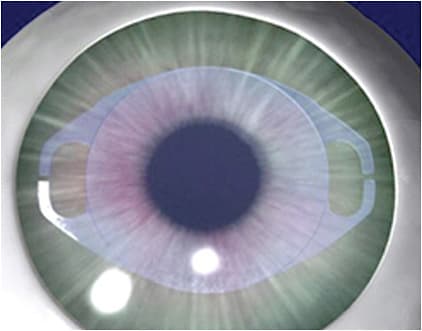
In a multicenter FDA study, 0 of the 629 eyes containing the EVO ICL developed anterior subcapsular opacities at 6 months.5,6 It has been suggested that the central port allows physiologic flow of aqueous, permitting bathing of the anterior capsule.7
The EVO+ ICL (for myopia—larger optic zone, and EVO+ Toric ICL for myopia/astigmatism—larger optic zone) has a larger optic diameter ranging from 5.0 mm to 6.1 mm. The EVO/EVO+ ICLs are FDA approved for the treatment of myopia ranging from -3.0 D to -15.0 D with ≤2.5 D of astigmatism, and are available in half diopter increments. They are also approved for the reduction of myopia ranging from -15.0 D to -20.0 D, with ≤ 2.5 D of astigmatism. The toric version is approved for the correction of astigmatism ranging from 1.0 D to 4.0 D
FUTURE APPLICATIONS STATESIDE
A potentially exciting use for phakic IOLs is the correction of presbyopia. Presbyopia affects 1.8 billion people worldwide—more than myopia and hyperopia.9 In presbyopes currently seeking spectacle independence, the surgical options include Laser Vision Correction (LVC) with monovision and Custom Lens Replacement (CLR). Both LVC and CLR are effective and safe procedures, but have certain limitations. Some patients cannot neuroadapt to monovision. Lens extraction in CLR can be associated with retinal detachment, especially in young, myopic eyes.10 Additionally, CLR eliminates any residual accommodative ability. Finally, in contrast to LVC and CLR, phakic IOL implantation is a reversible procedure.
Presbyopia-correcting pIOLs can offer similar vision in both eyes with preservation of the crystalline lens and residual accommodation. Additionally, a presbyopia-correcting IOL would delay extraction of the crystalline lens until a visually significant cataract develops. This would give patients access to future IOL technology.
Something else to consider: A presbyopic-correcting pIOL could be used as a piggyback lens. For example, if a patient who underwent cataract extraction with implantation of a monofocal IOL now desired spectacle independence, they may regret their lens choice. A piggyback presbyopic lens could be a great option for this type of patient. An IOL exchange may be challenging years later—especially if the patient already underwent a YAG capsulotomy.
The Need
The estimated worldwide prevalence of myopia (Manifest Refraction Spherical Equivalent, or MRSE, of ≥-0.50 D) and high myopia (MRSE ≥-5.00 D) is 22.9% and 2.7%, respectively; totaling about 1.4 billion myopes and 163 million high myopes. Due to a myriad of hypothesized factors, the prevalence of myopia and high myopia is estimated to increase to approximately 49.8% and 9.8%, respectively, by the year 2050.8 Based on population estimates, this projects to nearly 5 billion myopes and nearly 1 billion high myopes by 2050. The expected increase in global myopia will continue to make phakic IOLs a necessary tool for every refractive surgeon, and one that will become much more commonplace. CP
References:
- Pierné K, Malecaze F. Iris-fixated phakic intraocular lenses. In: Azar DT, Gatinel D, Ghanem RC, Taneri S, eds. Refractive Surgery, 3rd ed. Elsevier; 2019:401-410.
- Fechner PU, Haubitz I, Wichmann W, Wulff K. Worst-Fechner biconcave minus power phakic iris-claw lens. J Refract Surg. 1999;15(2):93-105.
- Staar Surgical. Staar Surgical announces approval by the FDA of the Visian Toric ICL for the correction of myopia with astigmatism. News release. September 13, 2018. Accessed March 10, 2023. https://www.staar.com/news/2018/staar-surgical-announces-approval-by-the-fda-of-the-visian-toric-icl-for-the-correction-of-myopia-with-astigmatism .
- Shimizu K, Kamiya K, Igarashi A, Shiratani T. Intraindividual comparison of visual performance after posterior chamber phakic intraocular lens with and without a central hole implantation for moderate to high myopia. Am J Ophthalmol. 2012;154(3):486-494.
- Multicenter clinical trial of a phakic Implantable Collamer Lens (ICL). January 2020. Updated March 3, 2023. Accessed March 10, 2023. https://clinicaltrials.gov/ct2/show/NCT04283149
- STAAR Surgical. EVO/EVO+ VISIAN Implantable Collamer Lens (EVO ICL) for Myopia directions for use. US Food and Drug Administration. Accessed March 10, 2023. https://www.accessdata.fda.gov/cdrh_docs/pdf3/P030016S035C.pdf
- Kawamorita T, Uozato H, Shimizu K. Fluid dynamics simulation of aqueous humour in a posterior-chamber phakic intraocular lens with a central perforation. Graefes Arch Clin Exp Ophthalmol. 2012;250(6):935-939.
- Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-1042.
- Fricke TR, Tahhan N, Resnikoff S, et al. Global prevalence of presbyopia and vision impairment from uncorrected presbyopia: systematic review, meta-analysis, and modelling. Ophthalmology. 2018;125(10):1492-1499.
- Colin J, Robinet A, Cochener B. Retinal detachment after clear lens extraction for high myopia: seven-year follow-up. Ophthalmology. 1999;106(12):2281-2284; discussion 2285.











