I know what some of you may be thinking, “Why should a corneal physician care about MIGS? Those are for glaucoma surgeons.” There are three reasons corneal surgeons should care:
- MIGS procedures can decrease the drop burden for our patients. For instance, a primary open-angle glaucoma (POAG) patient with preoperative IOP of 14 mmHg while using an anti-glaucoma drop can achieve a 16 mmHg IOP after MIGS (still the target range), and cease drop use. That is success. It can be hard for glaucoma patients to comply with their drop regimen due to the cost of medication, forgetfulness, and side effects, such as dry eye disease (DED), among other reasons. Poor compliance can lead to IOP fluctuation and, thus, glaucoma progression.
- Up to 60% of glaucoma patients have concomitant DED, whose development has also been related to the number of anti-glaucoma drops used.1-3 DED affects biometry reading accuracy, causing unsatisfactory visual outcomes after cataract surgery (CS).
- The surgical manipulations required to perform MIGS are well within the skill and scope of corneal surgeons.
With an understanding of the benefits of combining MIGS with cataract surgery, here I discuss the MIGS options and the related postoperative considerations. (See “Mastering MIGS”.)
Trabecular Microbypass MIGS
These devices carry the lowest risk of hyphema, as they do not require excising or cutting the trabecular meshwork (TM). Instead, they increase aqueous humor outflow through the TM into Schlemm’s canal, then to the episcleral venous network (which has its own pressure of 8 mmHg to 13 mmHg). Also, since there is no bleb creation, episcleral venous pressure prevents postoperative hypotony, and the effect on postoperative refractive error is insignificant.
Two trabecular microbypass devices are FDA approved for the mild-to-moderate (IOP in the middle to upper teens) glaucoma patient at the time of cataract surgery:
1. iStent inject W (Glaukos). The original iStent was approved by the FDA in 2012, and the iStent inject W was approved for POAG treatment in 2018. The iStent inject W procedure places 2 small stents (360 µm in diameter, 360 µm in height, with an 81 µm lumen) 2 clock hours apart through the nasal TM into Schlemm’s canal.
At 24 months in the pivotal US Investigational Device Exemption (IDE) Trial, 75.8% of iStent inject subjects had ≥20% reduction in unmedicated diurnal IOP (7.0 mmHg reduction from baseline). Mean medication burden decreased from 2.6 to 0.4 at 23 months; 84% of patients were drop free at 23 months.4 Another study revealed the iStent inject W increased outflow more than cataract surgery alone (Figure 1).2

Additionally, the iStent inject phase 3 trials demonstrated a higher percentage of patients showing meaningful improvements in quality-of-life scores, including OSDI and VFQ-25 when compared with patients who received cataract surgery alone.5 Patients who received the iStent inject showed a particular advantage in general health, ocular pain, and driving vision.
2. Hydrus Microstent (Alcon). FDA approved in 2018, this trabecular microbypass device is an 8 mm nitinol stent that bypasses the TM and scaffolds 3 clock hours of Schlemm’s canal. The Hydrus Microstent keeps the Schlemm’s canal from collapsing, allowing for access to the collector channels in that area 6 (Figure 2).
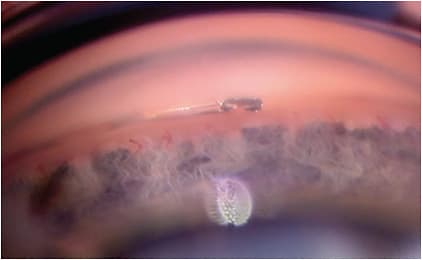
A 5-year trial demonstrated those patients in the Hydrus group needed fewer anti-glaucoma drops to achieve a 20% reduction of IOP from baseline.7 Through 5 years, rates of progressing to secondary surgical intervention were over 61% lower in the Hydrus group versus the CS alone group (2.4% in Hydrus versus 5.3% in CS only).
• iStent infinite (Glaukos). This 3-stent system, in which all stents are preloaded on one injector system, was FDA cleared in 2022 for patients who have failed prior medical and surgical intervention for glaucoma, and it is an implantable alternative that can be used in a standalone procedure.
In the pivotal trial, comprised of tough-to-treat patients who failed procedures, such as trabeculectomies, 20% or greater reductions in IOP were seen in 75% of iStent infinite patients, and 53% of patients achieved a 30% or greater decrease in IOP with the same or fewer anti-glaucoma drops.8
Canaloplasty/Viscodelivery
A canaloplasty MIGS approach involves a combination of microcatheterization and viscodilation, done using these devices: iTrack Microcatheter (Nova Eye Medical), Omni Surgical System (Sight Sciences), the iPrime (Glaukos), and Streamline (New World Medical). The iPrime achieved FDA 510(k) clearance in January 2022. No efficacy studies are currently available.
Catheterization of Schlemm’s canal breaks adhesions and herniations within the canal, improving drainage; then, injection of a high-molecular-weight ophthalmic viscosurgical device (OVD) further expands the TM, while simultaneously dilating the canal and the distal collector channels. This effect of stretching and opening the TM, canal, and collector channels can be observed with intraoperative OCT.
A single-center study completed in 2022 found that canaloplasty performed with the iTrack Microcatheter was effective in reducing IOP and medication dependence. Specifically, over 36 months, the data demonstrated that IOPs decreased from 20.5±5.1 mmHg preoperatively to 13.3±2.1 mmHg at 12 months, and the number of medications were reduced from 2.8±0.9 preoperatively to 1.1±1.1 at 12 months, with these numbers remaining stable at 24 and 36 months.9
The IOP and medication reductions were comparable whether the canaloplasty was performed as a standalone procedure or in conjunction with cataract surgery.
A recent study of canaloplasty using the Omni Surgical System in adult patients with open-angle glaucoma revealed statistically significant reductions in both IOP and in the use of IOP-lowering medications.10
In the high IOP group (>18 mmHg), mean IOP was reduced from 21.8 mmHg to 15.6 mmHg (P<.0001), and medications decreased from 2.2±0.9 to 0.9±1.1. The IOP-lowering effect lasted up to 42 months, and no difference in efficacy was noted in standalone intervention either in phakic or pseudophakic eyes.
Finally, an interim assessment of the Streamline Surgical System shows a mean IOP reduction of ≥20% from baseline achieved in 89.5% of eyes (17/19) at 6 months; from baseline through 6 months of follow-up to 14.7 (2.4) mmHg (P<0.001), representing an IOP decrease of 8.8 mmHg (36.9%). Additionally, 57.9% (11/19) of eyes were able to decrease dependence on IOP-lowering medications by at least one medication, and 42.1% (8/19) were medication free.11
Goniotomy
By removing or cutting a section of trabecular tissue, aqueous is given direct access to the collector channels and the distal outflow system. Excisional goniotomy can be performed using the Kahook Dual Blade (New World Medical), Sion (Sight Sciences), Trabectome (Microsurgical Technology), TrabEx and TrabEx Pro (Microsurgical Technology), or a bent ab interno needle goniectomy (BANG technique).
Cutting procedures like gonioscopic-assisted transluminal trabeculotomy (GATT) can be done with the Omni, iTrack, iPrime, or the iAccess or Streamline devices for micro-goniotomies. Per recent guidelines, to code goniotomy, one must cut or remove 90° of the TM.
MASTERING MIGS
Cornea and cataract surgeons have the skill set required to perform MIGS. As with any procedure, the view is the key to success!
- Start with gonioscopy in the office to get more comfortable with landmarks.
- During cataract surgery, start to use intraoperative direct goniolenses to view the angle even before starting your first MIGS. You will learn how much viscoelastic to use and how much pressure to place on the eye with the gonioprism. Too much pressure can cause striae.
- Practice turning the scope and head as well as holding the gonio lens on the cornea, (consider a hands-free lens).
- Start using a second instrument in your other hand to develop the muscle memory of working within the angle and so you can understand what it feels like to have a tool working in the angle.
- Avoid the perilimbal vessels with your incisions. They can cause bleeding, which can cloud the view under the gonio lens.
- When the TM is very light and hard to visualize, try removing viscoelastic or aqueous humor from the anterior chamber (AC) to lower the pressure in the eye. This will cause a reflux of blood in Schlemm’s canal and highlight the location of the TM. (Sometimes, you may not see that much pigment near the trabecular meshwork that you want to address, but you may see an area of TM that is more pigmented downstream. This will give you an indication of where the TM is located, and then you can follow it to the area where you want to start. (Many of the MIGS device companies help with training and offer in-office wet and dry labs to help you get started.)
Although I like to spare the TM as much as possible, goniotomy/trabeculotomy is a good option without having to worry about incorrectly placing a device. You need to, however, be careful of inadvertently causing a cleft if you are unsure of the anatomy. I do not stop blood thinners regardless of the MIGS procedure. The heme comes from reflux, not bleeding from tissue injury. If you do not cut ciliary body or iris tissue, you should not see a significant increased risk of heme in an anticoagulated patient. Another key to preventing postoperative hyphema is to pressurize the eye at the end of the case but then slowly decompress the eye by burping the wound. Do not decompress all at once; slowly release fluid over a minute or two. This will allow the AC and the elevated episcleral venous pressure to equilibrate.
A recent study examined outcomes after excisional goniotomy involving the Kahook Dual Blade. A total of 42 eyes were analyzed, of which 36 (85.7%) had mild-to-severe POAG. Mean IOP at baseline was 21.6±0.8 mmHg, and mean number of medications used at baseline was 2.6±0.2. At 12 months postop, mean IOP reduction from baseline was 3.9 mmHg, a reduction of 19.3% (P≤0.001), and medications were reduced by 0.3, a 12.5% reduction from baseline (P<0.05).12
A study on goniotomy using the Trabectome showed a statistically significant decrease in IOP (P<0.01) at final follow-up (average=18.35 months) of close to 23%, with a complication rate of 5.86% in 339 eyes. Additionally, this decrease was maintained up to 8 years postoperatively.13
Finally, a retrospective chart review study performed on all patients who underwent the BANG technique of goniotomy between July 2017 and June 2018, revealed mean IOP was 13.3±2.5 mmHg (P=3.6×10−7) on 0.5±0.8 topical glaucoma medications (P=0.01) at postoperative month 6.
A ≥20% reduction in IOP was achieved in 73% of patients, and 73% of patients needed ≥1 fewer glaucoma medications, while 73% of patients required no medications for IOP control. Further, 41% of those treated achieved IOP ≤12 mmHg.14
The other goniotomy devices mentioned received 510(k) clearance. Efficacy data is not available.
It’s worth mentioning that goniotomy has also shown good success in patients who have secondary POAG, such as pseudoexfoliation, pigment dispersion, and steroid-induced glaucoma. In these conditions, the disease process occurs primarily at the level of the TM. Therefore, removal of the TM makes sense.
In patients who have relatively small areas of peripheral anterior synechiae or in those who have relatively recent angle closure, goniosynechialysis followed by Kahook Dual Blade goniotomy can often be performed safely.
Whether cutting or removing TM, ophthalmologists can titrate how much of the TM they want to address, whether 90°, 180°, or 360°. The amount of TM addressed often depends on the severity of disease, drop burden on the patient, preoperative IOP, patient comfort level, and the patient’s anatomy.
Postoperative Considerations
Follow-up care for MIGS procedures is similar to that of cataract surgery. Patients are usually seen at 1 day, 1 week, 1 month, and then 3 months.
Common postoperative complications of MIGS are malposition or obstruction, and IOP elevation.Hyphema after surgery is most often transient and self-resolving. Stent obstruction is an infrequent complication that can lead to device failure. Early obstruction is typically due to device malposition, but chronic inflammation can lead to peripheral anterior synechiae and, later, obstruction. If the obstruction is clear, a YAG laser can remove it. Finally, watch for steroid spikes and cease prostaglandin use if the patient is using multiple preop glaucoma drops, though they can maintain aqueous suppressants.
One and Done
As illustrated above, combining MIGS with cataract surgery is an excellent means of tackling 2 ocular issues at once, saving the patient time, while preserving their vision and quality of life. At the end of the day, in keeping in mind the best interest of the patient, MIGS just makes sense. CP
MIGS DEVICES
Stent
- iStent inject, iStent inject W, and iStent infinite (Glaukos)
- Hydrus Microstent (Alcon)
Dilation
- Omni Surgical System (Sight Sciences)
- iPrime (Glaukos)
- iTrack Microcatheter (Nova Eye Medical)
- Streamline (New World Medical)
Excision/Cutting
- Trabectome (Microsurgical Technology)
- Kahook Dual Blade (New World Medical)
- TrabEx and TrabEx Pro (Microsurgical Technology)
- iAccess Trephine (Glaukos)
- Sion Surgical Instrument (Sight Sciences)
Subconjunctival MIBS
(Minimally invasive bleb surgery)
- Xen Gel Stent (Allergan)
Supraciliary
- Allopass (Iantrek)
Cyclophotocoagulation
- Endo optiks endoscopic cyclophotocoagulation (BVI)
- Cyclo G6 and MicroPulse P3 (Iridex)
References:
- Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29(6):618-621.
- Erb C, Gast U, Schremmer D. German register for glaucoma patients with dry eye. I. Basic outcome with respect to dry eye. Graefes Arch Clin Exp Ophthalmol. 2008;246(11):1593-1601.
- Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350-355.
- Samuelson TW, Sarkisian SR Jr, Lubeck DM, et al; iStent inject Study Group. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126(6):811-821.
- Samuelson TW, Singh IP, Williamson BK, et al. Quality of life in primary open-angle glaucoma and cataract: an analysis of VFQ-25 and OSDI from the iStent inject pivotal trial. Am J Ophthalmol. 2021;229:220-229.
- Gong H, Francis A. Schlemm’s canal and collector channels as therapeutic targets. In: Samples JR, Ahmed I, ed. Innovations in Glaucoma Surgery. Springer; New York, NY: 2014:3-25.
- Ahmed IIK, De Francesco T, Rhee D, et al; HORIZON Investigators. Long-term outcomes from the HORIZON randomized trial for a Schlemm’s canal microstent in combination cataract and glaucoma surgery. Ophthalmology. 2022;129(7):742-751.
- Sarkisian SR Jr, Grover DS, Gallardo MJ, et al; iStent infinite Study Group. Effectiveness and safety of iStent trabecular micro-bypass for uncontrolled glaucoma. J Glaucoma. 2023;32(1):9-18.
- Gallardo MJ. 36-month effectiveness of ab interno canaloplasty standalone versus combined with cataract surgery for the treatment of open-angle glaucoma. Ophthalmol Glaucoma. 2022;5(5):476-482.
- Ondrejka S, Körber N, Dhamdhere K. Long-term effect of canaloplasty on intraocular pressure and use of intraocular pressure-lowering medications in patients with open-angle glaucoma. J Cataract Refract Surg. 2022;48(12):1388-1393.
- Lazcano-Gomez G, Garg SJ, Yeu E. Kahook MY. Interim analysis of STREAMLINE surgical system clinical outcomes in eyes with glaucoma. Clin Ophthalmol. 2022;16:1313-1320.
- ElMallah MK, Berdahl JP, Williamson BK, et al. Twelve-month outcomes of stand-alone excisional goniotomy in mild to severe glaucoma. Clin Ophthalmol. 2020;14:1891-1897.
- Bendel RE, Patterson MT. Long-term effectiveness of trabectome (ab interno trabeculectomy) surgery. J Curr Glaucoma Pract. 2018;12(3):119-124.
- Shute T, Green W, Liu J, Shaybani A. An alternate technique for goniotomy: description of procedure and preliminary results. J Ophthalmic Vis Res. 2022 29;17(2):170-175.









