Case Associated with Contaminated Eye Drops Seen Three Months Prior to Warning
A case study in Antimicrobial Agents and Chemotherapy describes a patient who presented in November 2022 with a drug-resistant Pseudomonas Aeruginosa (PA) corneal ulcer from the same eye drop the Centers for Disease Control (CDC) issued a warning about in February 2023.1 That eye drop was EzriCare Artificial Tears, which was produced at a facility in India that the FDA subsequently determined had several sterilization issues.
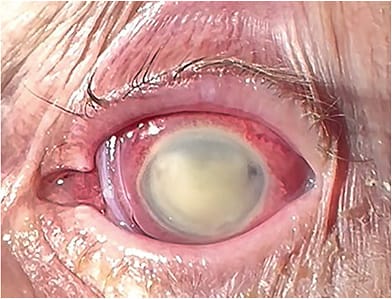
“I’ve never recovered it [the drug-resistant PA] from an eye,” explained the study’s first author, Morgan Morelli, MD, who is also an infectious disease fellow at the UH Cleveland Medical Center, in a press release about the study. “[Finding the right diagnosis] required a lot of thinking and digging to figure out what was going on, and we never thought [the drug-resistant PA] was related to a global manufacturing issue.”
Since the CDC’s February 2023 warning, Delsam Pharma’s Artificial Tears, and Delsam Pharma’s Artificial Ointment, also produced at the aforementioned facility, have also been implicated in causing the drug-resistant PA.
At press time, 81 patients in 18 states have developed the infection, including 14 who have lost their vision, 4 who have undergone enucleation, and four who have died, according to the CDC.
Reference:
- Antimicrob Agents Chemother. 2023 May 11;e0027723. doi: 10.1128/aac.00277-
Small Study Appears to Confirm Marvel Character’s Superpower
How amazing would it be if a superhero’s one or more powers were real? Pretty amazing, right? One recent study proves just that. Specifically, a study in Neuroimage reveals that when one eye is covered, hearing is boosted. This finding appears to confirm Daredevil’s amplified-hearing superpower, which, in the Marvel comic, happened after the defense attorney-by-day, masked-vigilante-by-night was blinded by a childhood accident.

For the study, researchers from Italy’s IMT School for Advanced Study’s Molecular Mind Laboratory placed an eye patch on 20 subjects, and requested they count flashes of light, while hearing beeping for roughly 2 hours.
An electroencephalogram registered the brain’s activity during the experiment, so the researchers could assess the neural response to both the visual and auditory stimuli after removing the eye patch.
The results showed that occluding one eye increased neural response to sounds.
Additionally, after removing the eye patch, the neural response became more sensitive to the information provided by the previously covered eye.
“While many studies have demonstrated the plasticity of the senses after prolonged sensory deprivation, as in the case of blindness and deafness, these findings unveil the high degree of plasticity and interdependence of the senses and the profound impact that sensory experience continuously exerts on our brain,” explained Davide Bottari, researcher at the IMT School and the study’s principal investigator, in a press release regarding the study.
The study’s researchers conclude the findings may play a role in creating interventions for “different kinds of diseases and disabilities, such as amblyopia.”
While the study’s authors do not mention that the findings may play a role in increasing one’s accuracy with martial arts, Daredevil’s nemesis Bullseye can certainly attest to that in the Marvel comic.
ALSO NOTEWORTHY:
- Alcon’s vice president of professional affairs, Rick Weisbarth, OD, has retired. Dr. Weisbarth began his career with Alcon in 1982, when he joined CIBA Vision as a clinical research optometrist. He will continue to serve as president of the Alcon Children’s Vision Center, according to a press release. In other news, Alcon launched its Alcon Experience Academy app to help optimize eye care professionals’ (ECPs) digital learning experiences, the company says. The customizable global platform offers ECPs courses of varying levels in 17 languages, as well as newly launched podcasts and discussion forums on eye care topics. The app can be downloaded from the Apple App Store or Google Play.
- Bausch + Lomb Corporation (B+L) and Novaliq GmbH announced that the FDA approved perfluorohexyloctane ophthalmic solution, formerly known as NOV03 (Miebo), for the treatment of the signs and symptoms of dry eye disease (DED). The drug directly targets tear evaporation. The most common adverse reactions experienced with Miebo were blurred vision (1.3% to 3%) and eye redness (1% to 3%). In other news, B+L announced the U.S. launch of the StableVisc cohesive ophthalmic viscosurgical device (OVD), and the TotalVisc Viscoelastic System. StableVisc helps maintain space in the eye’s anterior chamber to allow extraction and replacement of the clouded natural lens. The TotalVisc Viscoelastic System is comprised of both the StableVisc and ClearVisc, a dispersive OVD, and protects ocular tissue during the surgical procedure.
- BioTissue was certified by Great Place to Work in the United States. Great Place to Work recognizes companies based entirely on employee feedback received through independent assessments, according to the press release. Specifically, a total of 87% of BioTissue employees affirmed that BioTissue is a great place to work and feel empowered. Further, more than 90% of the company’s employees said they take great pride in their work.
- BostonSight introduced Lensy, a 3D plush scleral lens as the newest ambassador for the company. Lensy is the main character in the picture book, “Lensy: A Scleral Lens Story,” which is about the basics of scleral lenses and follows her friend, Sophie Sheep, who needs scleral lenses due to extreme dry eyes. A limited number of Lensy plushies and printed books are available for donation to pediatric patients at Prevention Research Center Network sites via a fundraising campaign: Give (or Get!) the Gift of Lensy. Information is available at www.bostonsight.org/give-lensy .
- BVI completed enrollment of its U.S. Investigational Device Exemption (IDE) clinical study for its latest hydrophobic trifocal IOL, Finevision HP. Patients are now being followed to evaluate the safety and performance of the IOL, with the results to be used for submission to obtain marketing approval in the United States, according to the press release.
- Contamac appointed John Clamp as director, Strategic Projects. In this role, Mr. Clamp will drive long-term strategic initiatives for the business in partnership with the company’s Research and Development group and operations team, according to the press release. Mr. Clamp has developed software ranging from full system-wide applications, through to full contact lens 3D design and creation of machining files, according to the press release.
- Euclid Vision Corporation is joining forces with Visionary Optics to host specialty contact lens dinner discussions around the United States. The program, “Patient Selection to Practice Growth: A Guide to Specialty Contact Lenses,” will bring eye care practitioners together to learn from industry experts and network with like-minded professionals about how to identify patient selection criteria for both orthokeratology and scleral lenses, develop a practice growth strategy, and troubleshoot common issues, according to the press release. The events will occur in Chicago (June 5), Boston (July 13), Southern California (Sept. 20), and Houston (Nov. 2). To learn more or to register, visit www2.euclidlenses.com/guide-to-specialty-contact-lenses-event .
- Eversight announced the availability of Descemet’s membrane endothelial keratoplasty (DMEK) tissue preloaded in EndoGlide. Preloaded DMEK EndoGlide utilizes an endothelium-in or trifold-loading method. Using an anterior chamber maintainer for infusion, the graft is pulled into the eye with specialized forceps through a 2.65 mm incision, according to the company.
- Eyedea Medical was awarded a $1,000,000 phase 2 Small Business Innovation Research grant from the National Science Foundation. The grant will be used for the development and commercialization of the company’s proprietary corneal tissue preparation devices, DescePrep and DesceCleave. These devices provide a simple, efficient, and standardized separation of layers of the cornea to increase access to and adoption of corneal transplant techniques, including Descemet’s Membrane Endothelial Keratoplasty and the Deep Anterior Lamellar technique, according to the press release.
- Eyenovia announced that the FDA approved Mydcombi (tropicamide and phenylephrine hydrochloride ophthalmic spray 1%/2.5%) for inducing mydriasis for diagnostic procedures and in conditions in which short-term pupil dilation is desired. This fixed-dose combination drop is the first product using Eyenovia’s proprietary Optejet device to be approved by any regulatory authority. For more information, visit https://eyenovia.com/pipeline/mydriasis .
- The FDA recently released a safety communication regarding the use of amniotic fluid eyedrops. Specifically, the communication warned that manufacturers of said drops are “marketing and distributing amniotic fluid eye drops to treat, mitigate, or cure diseases or conditions, such as dry eye disease, without the required premarket review and approval.” Additionally, the government agency stated that such manufacturers have been notified. For additional information, visit www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/public-safety-notification-amniotic-fluid-eyedrops .
- Haag-Streit launched a corneal ectasia display for the Eyestar 900 swept-source-based precision OCT analyzer. The display features OCT-based topography for elevation information of the cornea’s front and back surface, as well as pachymetry maps and profiles, Belin ABCD grading, and complementary parameters to aid in identifying signs of ectatic changes, and the first signs of keratoconus, according to Haag-Streit.
- Harrow named Mark Mannenbach, PhD, RPh, its head of regulatory affairs and pharmacovigilance. In this position, Dr. Mannenbach will be responsible for overseeing and managing all regulatory submissions and strategy related to the company’s portfolio of new and existing products.
- Heidelberg Engineering named Dr. Hendrik Spahr and Dr. Dierck Hillmann winners of the 2023 Heidelberg Engineering Xtreme Research Award for their research on holographic OCT. Their research, entitled “Towards holographic OCT for clinical applications,” demonstrates what becomes possible when applying techniques from digital holography and astronomy to a laboratory, full-field OCT system. The researchers developed advanced data-processing techniques to correct for disturbances that are unavoidably introduced by eye motion, according to the press release.
- Johnson & Johnson Vision named Lori Tierney as president, Americas; Jacqueline Henderson as president; and Christoph Vonwiller as president, Asia Pacific. The 3 executives will lead the end-to-end vision business in their respective regions, driving strategy, commercial excellence, and organizational alignment of the vision care and surgical vision businesses, according to the press release. In other news, Johnson & Johnson Vision’s Elita femtosecond laser received FDA 510(k) clearance for the creation of LASIK flaps. The Elita femtosecond laser flap features a low energy per pulse, small spot size (1 µm), an intuitive user interface, modular design, and a quick system startup (less than 5 minutes), according to the company.
- Kala Pharmaceuticals, Inc’s KPI-012 for the treatment of persistent corneal epithelial defect received Fast Track designation from the FDA.
- Levation Pharma Ltd. received FDA clearance to initiate a phase 1/2 clinical trial of its of LEV102 for the treatment of acquired blepharoptosis in adults. LEV102 is a proprietary aqueous gel comprised of several penetration enhancers and oxymetazoline as the active pharmaceutical ingredient. Oxymetazoline is an alpha-adrenergic agonist that activates the receptors on Mueller’s muscle in the upper lid. (Mueller’s is the involuntary muscle that raises the lid; for example, when one is scared.)
- Lions Eye Institute for Transplant and Research and SightLife are now Lions World Vision Institute (LWVI). As one nonprofit organization, it offers a broader, worldwide network of eye banks, physicians, researchers, and community-based services, according to the press release. LWVI’s services are grounded in three focus areas: (1) Donation and Transplant Services, (2) Research and Innovation, and (3) Prevention & Education. The organization is headquartered in Tampa, Florida.
- Medical Consulting Group (MCG) and Corcoran Consulting Group (CCG) announced a definitive merger agreement. The former provides consulting services to ophthalmic practices and companies, and the latter specializes in billing, coding, and reimbursement issues for ophthalmology and optometry practices, according to the press release.
- Norlase, an ophthalmic laser manufacturer says it closed its biggest investment round thus far, with $11 million USD in funding by London-based firm West Hill Capital. The funding comes on the heels of the FDA clearance and CE Mark approval of the company’s Echo pattern laser. In addition to Echo, the Norlase product portfolio includes the portable Leaf single-spot laser and Lion laser indirect ophthalmoscope.
- NovaBay Pharmaceuticals announced a partnership with Eyeganics to sell OTC Organic Tears (0.2% organic glycerin) on www.avenova.com and through Avenova’s physician-dispensed channel. Organic Tears are formulated using three ingredients: (1) organic glycerin (oil from organic vegetables), (2) salt, and (3) water. The product is offered in the 10 ml size for $29.00.
- Ocuphire Pharma appointed Rick Rodgers as interim CEO and president. Mr. Rodgers succeeds Mina Sooch. The company has retained an executive search firm to assist in identifying a permanent CEO.
- OKYO Pharma Limited activated the first clinical trial site in the United States for a phase 2, multi-center, randomized, double-blinded, placebo-controlled trial. Specifically, the trial will evaluate the efficacy and safety of OK-101 ophthalmic solution, a lipid conjugated chemerin peptide antagonist of the ChemR23 G-protein coupled receptor, typically found on immune cells of the eye responsible for the inflammatory response, in subjects who have dry eye disease.
- Selagine, Inc., entered a research, development, and sublicense agreement with Grifols, a creator of plasma-derived medicines, for the development and commercialization of an immunoglobulin eye drop for dry eye disease. The eye drop contains functional antibodies generated from human plasma, according to the press release. Selagine is a spin-out company from the University of Illinois at Chicago.
- Sight Sciences, Inc. appointed Alison “Ali” Bauerlein its CFO and treasurer, effective April 3, 2023. She joins Sight Sciences from Inogen, Inc, a medical technology company she co-founded in 2001 that offers respiratory products for use in the home care setting. She served as CFO for this company from 2009 to 2021.
- Synchrony’s CareCredit partnered with CoFi, a technology provider in the vision industry and multi-party patient payment platform. Through this partnership, the CareCredit payment solution will be integrated into the platform for the more than 2,000 ophthalmologists, optometrists, and surgery centers on CoFi’s network. This will enable patients to use a CareCredit credit card to finance their consolidated care costs, according to the press release.
- Tarsus Pharmaceuticals, Inc. launched “Don’t Freak Out. Get Checked Out,” a disease-education campaign designed to encourage patients who may have Demodex blepharitis visit their eye care provider for an eyelid check. The “Don’t Freak Out. Get Checked Out.” website, www.EyelidCheck.com , features tools and educational resources, including patient testimonials, information about joining a Demodex blepharitis community, social media channels, and a quiz on differentiating factors of potential causes of eyelid discomfort.
- The Tear Film & Ocular Surface Society (TFOS) announced its global workshop report, “A Lifestyle Epidemic: Ocular Surface Disease,” will be published this quarter in The Ocular Surface journal. “This new TFOS report focuses on the direct and indirect impacts that everyday lifestyle choices and challenges have on ocular surface health — from screen time, to our beauty routines, to our nutrition, to where we live,” stated Amy Gallant Sullivan, executive director of TFOS.
- Visus Therapeutics, Inc., appointed a new senior vice president of business development and marketing, Jehan Tamboowalla. Mr. Tamboowalla is responsible for identifying and executing strategic partnerships and collaborations, and leading commercial activities for assets in clinical development. In other news, the company presented key topline data from its phase 3 BRIO-I clinical trial. The study, evaluating the safety and efficacy of Brimochol PF, a preservative-free ophthalmic solution for the treatment of presbyopia, met its primary and secondary endpoints. This was achieved based on the proportion of subjects achieving >15 ETDRS letter gain in binocular near visual acuity without a loss of ≥5 letters at distance across all time points.
- Zeiss Medical Technology announced the FDA approval of the CT Lucia IOL. The aspheric, monofocal, single-piece C-loop IOL features the Zeiss Optic Asphericity Concept. This is designed to compensate for a wide range of spherical aberrations and, while optimizing visual outcomes in the event of potential decentration and lens misalignments, according to the press release. CP








