Since Eduard Zirm performed the first successful human penetrating keratoplasty (PK) surgery in 1905,1 corneal transplant surgery has become a standard surgical treatment for corneal disease. One of the main indications for PK prior to 2000 was endothelial disease.
After 1990, Fuchs’ endothelial dystrophy became a leading indication for PK. With the development of Descemet’s membrane automated endothelial keratoplasty (DSAEK) and Descemet’s membrane endothelial keratoplasty (DMEK) in early 2000, along with improved deep anterior lamellar keratoplasty (DALK) techniques at the same time, PK numbers started to decline after 2005. This is when replacing the diseased layer of the cornea became feasible (see Figure 1).
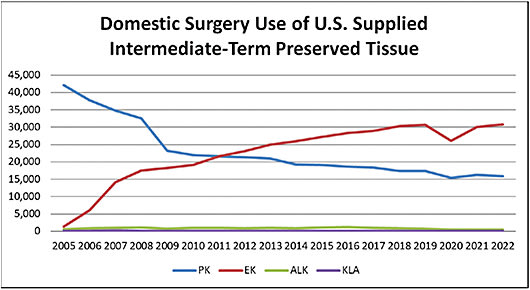
The 2022 Eye Bank Association of America (EBAA) statistical report shows that selective transplants have overtaken PK in the surgical treatment of corneal disease.2 In 2022, 30,812 endothelial keratoplasty (EK) procedures were performed in the United States, nearly double the number of PK procedures at 15,835 (Table 1). Of note, 31,952 PK procedures were performed in 2005 compared to 1,429 EK procedures that year.2
DSAEK
All EK procedures in the United States currently use donor tissue prepared by a microkeratome. In Descemet’s membrane endothelial keratoplasty, the donor tissue is prepared with manual dissection by the surgeon. DSAEK entered the picture when researchers noted that a thin layer of posterior stroma with attached Descemet’s membrane could be peeled off from a microkeratome blade that was used for refractive surgery without harming donor endothelium,3,4 and that an air bubble could be used to attach the donor tissue to the posterior stroma without the need for sutures.
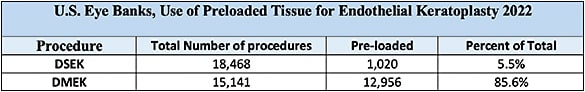
When DSAEK was in its infancy, surgeons used their own microkeratome for donor tissue preparation. Eye banks eventually started cutting the donor tissue with microkeratomes for the surgeon, reducing the responsibility of the surgeon for possible tissue injury from cutting and possibly obviating a cancelled case. Eye banks now prepare most EK tissue for surgeons in the United States (see Table 2).
- Procedure. The DSAEK procedure involves tissue preparation with a microkeratome by a surgeon or the eye bank. The donor endothelial lenticule is delivered into the eye through a 4 mm to 5 mm corneal incision. The donor tissue can be pushed or pulled into the anterior chamber, either using instruments to fold and insert the tissue or an injection tube for delivery. A few sutures are placed in the wound. The anterior chamber is filled with air or sulfur hexafluoride (SF6) to appose the donor stroma to the recipient stroma. Partial release of air is performed to reduce the risk of pupillary block postoperatively.
- Indications. DSAEK is the preferred procedure in patients who have corneal edema, that compromises the surgeon’s view through the microscope. The procedure is better suited in more complicated situations, such as in patients with dilated pupils, glaucoma filters, high myopic long eyes, aphakia, and large iridectomies.
- Benefits. Compared to PK, DSAEK results in faster visual rehabilitation, less-induced astigmatism, less suture-related complications, and less risk of wound dehiscence postoperatively. Additionally, both ametropia and intraoperative complications are drastically reduced by leaving the patient’s Bowman’s layer, the main refractive layer of the eye, and stroma untouched.
- Possible complications. Pupillary block from the air or gas pushing the iris forward over the trabecular meshwork may occur over the first 48 hours. Interface infections, dislocation of the tissue, endothelial rejection or failure, and interface haze may occur in the days or weeks after surgery.
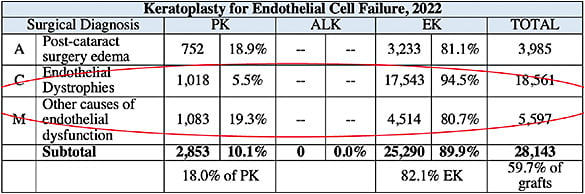
- Utilization. DSAEK was the first EK procedure to reach wide acclaim and has been tracked by the EBAA starting in 2005. Of note, in 2022, 94.5% of Fuchs’ endothelial dystrophy patients were treated with either DSAEK or DMEK procedures (see Table 3).
DMEK
- Procedure. The DMEK procedure, pioneered by Gerrit Melles, transplants Descemet’s membrane and endothelium without a stromal carrier. The DMEK donor tissue can be prepared by the surgeon or the eye bank, much like with DSAEK.With DMEK, the DM/endothelium complex is peeled away from the donor stroma, stained with trypan blue, and placed in a glass injector or IOL cartridge.
A total of 95% of DMEK tissue is prepared by eye banks and delivered to the surgeon, again reducing the surgeon’s responsibility for mishaps in tissue preparation (see Table 3). The tissue is injected through the small corneal incision, and fluid waves are used to unroll and center the tissue. Air or sulfur hexafluoride (SF6) are used to fill the anterior chamber and appose the donor tissue to the recipient stroma. An inferior peripheral iridectomy can be used to lower the risk of pupillary block from the air. The air or gas slowly dissipates over the week, and vision improves. - Indications. DMEK is preferred for many cases of corneal endothelial disease in situations when the cornea view is not compromised and the comorbidities mentioned for DSAEK are not an issue. The most common indications include Fuchs’ endothelial dystrophy, pseudophakic bullous keratopathy, and failed grafts with no stromal scarring and no astigmatism.
- Benefits. DMEK shares the same advantages that DSAEK has over PK but affords the advantages of quicker vision recovery, less induced ametropia, reduced endothelial rejection, and avoidance of suture-related complications.5DMEK gained popularity in 2013, following published reports of better vision and faster visual rehabilitation compared to DSAEK. Because the residual stroma transplanted by DSAEK lenticules can create an interface that limits best-corrected visual acuity (BCVA), more patients with DMEK are likely to have a BCVA up to 20/20 compared to DSAEK and PK. DMEK does not have a stroma-to-stroma interface, as with DSAEK, and lacks the multiple sutures needed in PK wound closure. Patients are typically tapered off steroids sooner after DMEK than with PK and DSAEK, reducing postoperative steroid-induced elevated IOP.
- Possible complications. DMEK has a steeper learning curve, higher risk of primary graft failure, and a higher risk of tissue dislocation and a higher incidence of re-bubble than DSAEK. Both DMEK and DSAEK carry a risk of interface infections.
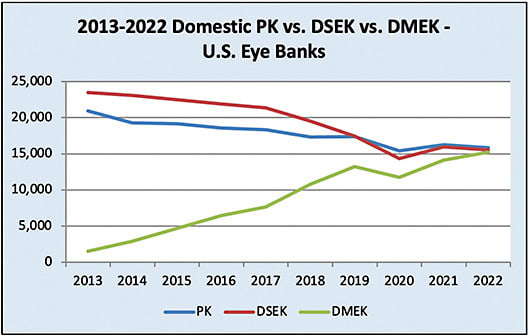
- Usage. According to the 2022 EBAA Statistical Report, the number of DMEK procedures in the United States was comparable to DSAEK for the first time in 2022, with 15,544 DSAEK procedures and 15,248 DMEK procedures. By comparison, in 2012 there were 22,301 DSAEK procedures and 748 DMEK procedures (Figure 2).
DALK
DALK became feasible when the trephine blade was developed to cut PK wounds, enabling a deep circular wound to be cut in the donor and host cornea. Early DALK procedures were performed by trephining deeply into the stroma, then dissecting across the cornea transversely, and removing as much stroma as possible. As instrumentation and techniques for this procedure improved, outcomes for DALK have improved worldwide.
- Procedure. The DALK procedure requires removal of as much of the host stroma as possible, and then suturing in a donor cornea. Techniques include manual dissection with a lamellar blade; “grip and rip,” which uses special forceps to “grip” the peripheral stroma and uses a pulling maneuver to separate stroma from the underlying Descemet’s (the rip portion); and the big-bubble DALK, which uses a big-bubble technique to separate the Descemet/endothelial layer from the adjacent stroma. The “big bubble,” when accomplished, reduces the amount of stroma left in the cornea and minimizes interface scarring.6,7 (See “DALK: Performing Big-Bubble Keratoplasty,” at bit.ly/DALKCPBigBubble .)
- Indications. The main indication for DALK worldwide remains stromal disease with a healthy recipient endothelium. Suitable diagnoses for DALK include keratoconus, pellucid marginal degeneration, corneal scars from infection or trauma with healthy endothelium, and stromal corneal dystrophies. The procedure can be used for tectonic purposes in peripheral corneal thinning diseases, such as Terrien’s marginal degeneration, peripheral ulcerative keratitis, and Mooren’s ulcers, once inflammation is under control.
- Benefits. The main advantage of DALK over PK is the preservation of the healthy host endothelium, independent of the donor endothelium. Endothelial rejection is eliminated or significantly reduced. since the healthy recipient endothelium is preserved.Another advantage is the avoidance of an open-sky procedure, which reduces the risk of intraoperative expulsive choroidal hemorrhages, glaucoma, cataract, endophthalmitis, and retinal complications compared to PK.
- Possible complications. DALK has a longer intraoperative time, a steeper learning curve for surgeons, and a risk of interface complications not seen with PK. Other complications include interface infections, interface fluid, pupillary block, stromal rejection, and suture-related risks.
- Usage. The number of lamellar keratoplasty procedures in the United States has been very small and essentially unchanged since EBAA statistics started (see Figure 1, Table 1, Table 4).
The Need Remains
The corneal surgeons of today who have helped pioneer the advent of selective keratoplasty, such as Melles, Terry, and Anwar, have helped improve results and reduce complications of corneal transplant surgery.
Selective keratoplasty surgery today can be more effective, more efficient, and safer than full-thickness keratoplasty.
With corneal blindness still a major impediment to sight worldwide and overall keratoplasty procedures on the rise, this trend toward specific procedures to replace specific corneal layers, or selective keratoplasty, is likely to continue. CP
References:
- Zirm E. [A successful total keratoplasty]. Graefes Arch Ophthalmol. 1906;64:580-593.
- 2022 Eye Banking Statistical Report. Eye Bank Association of America. Accessed June 5, 2023. https://restoresight.org/members/publications/statistical-report/
- Filatov VP. Transplantation of the cornea from preserved cadavers’ eyes. Lancet. 1937;229(5937):1395-1397.
- Castroviejo R. Keratoplasty: Comments on the technique of corneal transplantation. Source and preservation of donor’s material. Report of new instruments. Am J Ophthalmol. 1941;24(1):1-20.
- Deng SX, Lee WB, Hammersmith KM, et al. Descemet membrane epithelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2018;125(2):295-310.
- Anwar M, Teichmann KD. Big-bubble technique to bare Descemet’s membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002;28(3):398-403.
- Anwar M, Teichmann KD. Deep lamellar keratoplasty: surgical techniques for anterior lamellar keratoplasty with and without baring of Descemet’s membrane. Cornea. 2002;21(4):374-383.









