Corneal blindness is a leading cause of vision loss worldwide, often requiring a corneal transplant (keratoplasty) for treatment.1 In developed countries, partial thickness corneal transplants have now become the most often performed corneal transplants with endothelial keratoplasty (EK) being the most common.2,3 Descemet’s membrane endothelial keratoplasty (DMEK) and Descemet’s stripping automated endothelial keratoplasty (DSAEK) have significantly improved visual outcomes for patients. However, these methods are limited by the availability of donor tissues.
A recent survey globally quantified the shortage of corneal graft tissue and concluded that there was only one cornea available for every 70 needed worldwide.4 It is clear that efforts to develop alternative and/or complementary solutions, such as corneal bioengineering are of interest. This article explores the development and clinical application of 3D-printed ex vivo-expanded endothelial keratoplasty, a promising advancement that addresses these limitations.
The Rationale
The Precise Vision endothelial keratoplasty (PVEK) approach, developed by Precise Bio, aims to mitigate this problem by utilizing advanced 3D printing technology to create high-density human endothelial cell implants supported by a human collagen scaffold. This innovation not only ensures a consistent supply of grafts but also maintains high cell viability and functionality.
Development of PVEK
The development of PVEK involves several key technological advancements:
1. Cell expansion technology. Corneal endothelial cells (CECs) are isolated from donor corneas and expanded using a proprietary media expansion protocol, enhancing cell proliferation while maintaining phenotype integrity.
2. Biomaterials technology. A human collagen scaffold, important for CEC support, is developed through collaborations with GMP-compliant collagen manufacturers. The scaffold is engineered to be a few microns thick, allowing for easy manipulation during surgery.
3. 3D printing technology. The Precise Ophthalmology Printer is utilized to print a monolayer of human CECs on the human collagen scaffold. This process ensures uniform cell distribution and high cell density, crucial for the implant’s success.
Clinical Implementation of PVEK
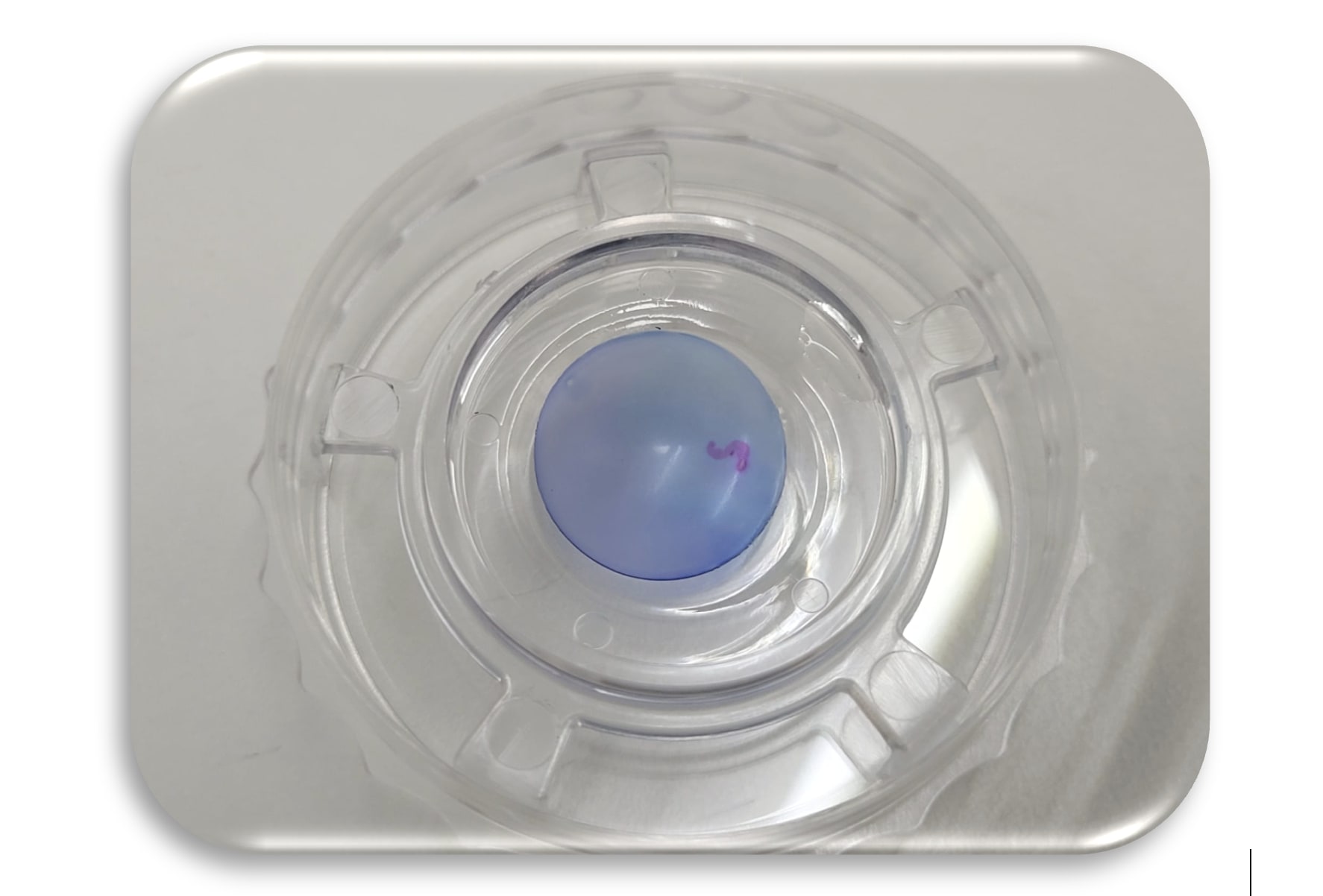
Preparation for PVEK implantation involves rigorous quality control to ensure product consistency (Figure 1). The surgical procedure itself is designed to be straightforward, minimizing manipulation and reducing the learning curve for surgeons:
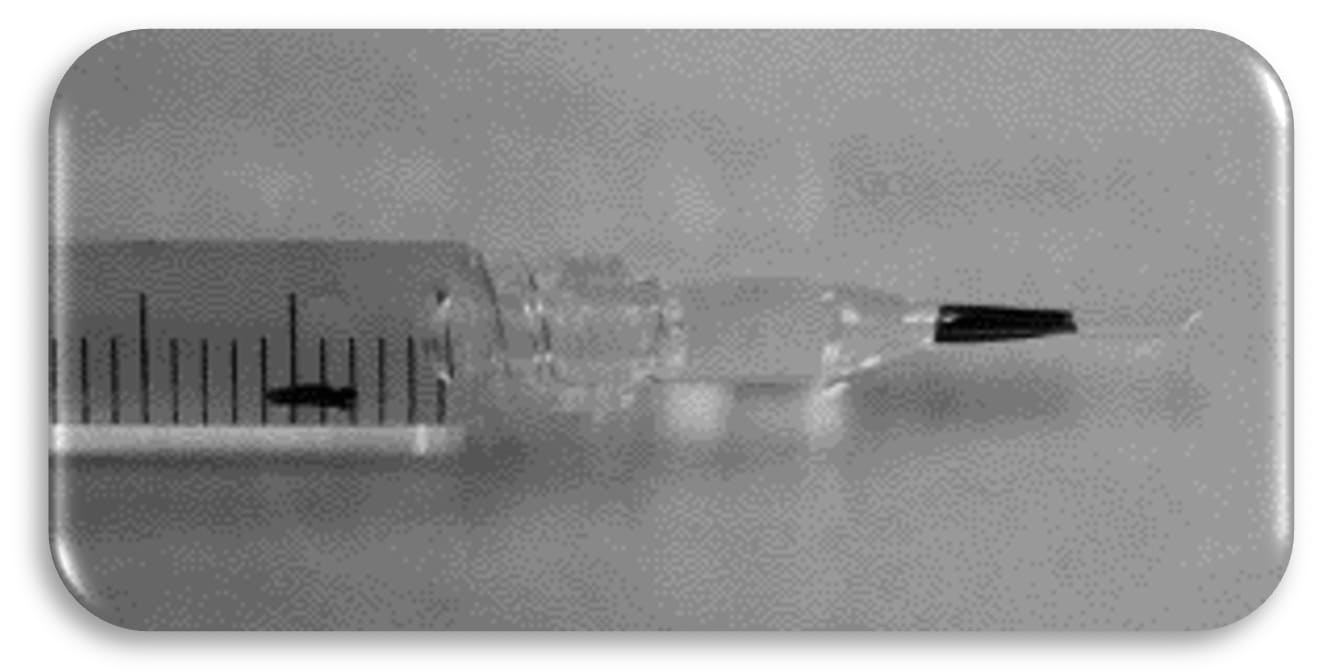
1. Preparation: The PVEK implant is preloaded in a glass injector (similar to a DMEK), ready for immediate use (Figure 2). This eliminates the need for additional handling, reducing the risk of cell damage.
2. Surgical procedure: Following a standard descemetorhexis as performed in DSAEK/DMEK, the implantation process involves a small incision through which the PVEK is inserted and positioned. The collagen scaffold unfolds relatively easy (similar to a DSAEK) allowing for easy manipulation and centering (see video: https://youtu.be/Ke8Ts14IMb8). Adherence of the graft is then achieved via standard endotamponade with air or gas (SF6/C3F8) similar to a DSAEK/DMEK.
3. Postoperative care. Postoperative management includes regular follow-ups to monitor implant integration and corneal health. Topical treatment includes antibiotics and steroids to prevent infection and inflammation.
Preclinical Studies and Results
Extensive preclinical testing and GLP studies have demonstrated the efficacy and safety of PVEK implants in laboratory environments and animals:
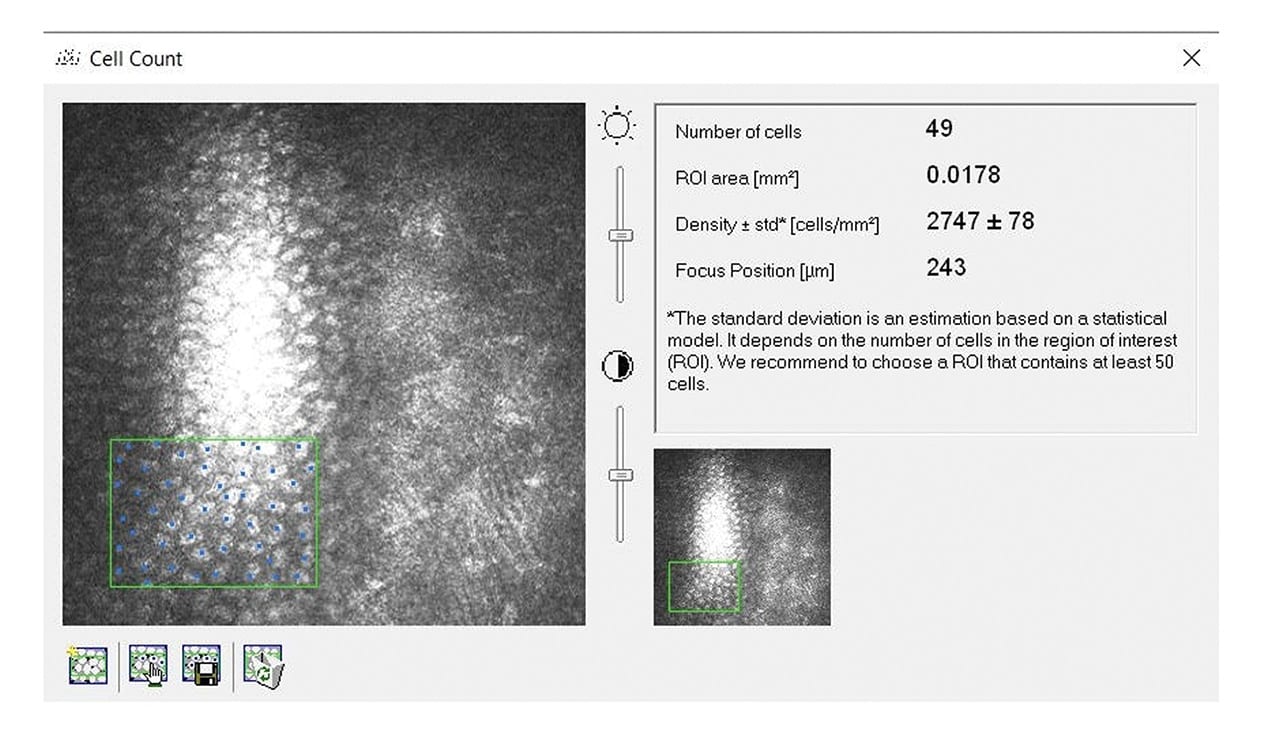
1. Preclinical studies. Initial tests in rabbit models showed successful implantation and maintenance of clear, compact corneas with high cell viability. These studies provided crucial data on the implant’s performance in vivo (Figure 3).
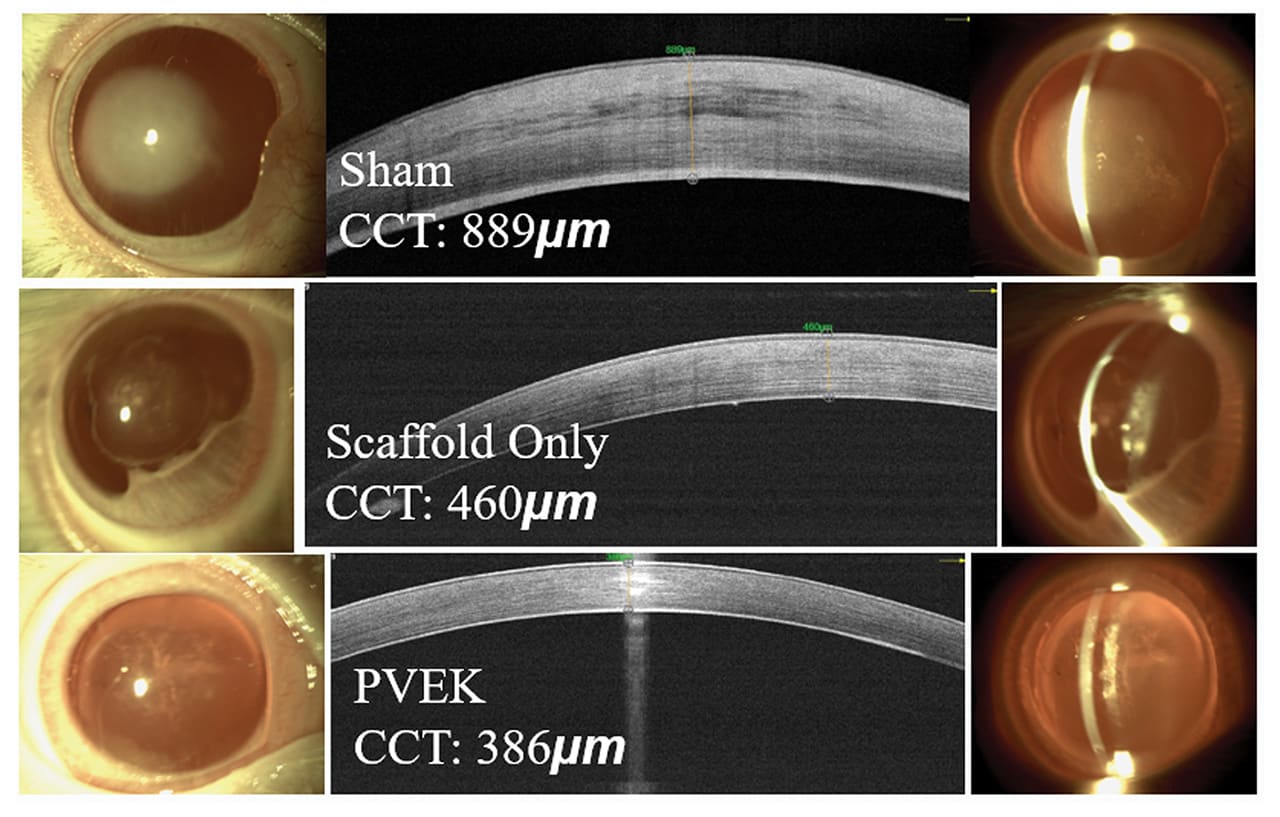
2. GLP study results. The GLP study involved 64 rabbits divided into three groups. The sham group, consisting of 16 animals, underwent descemetorhexis only, serving as the control group to provide baseline data on corneal healing without any implant. The scaffold-only group included 22 animals that received the collagen scaffold without endothelial cells, isolating the effects of the scaffold alone. The PVEK group, composed of 26 animals, received the full PVEK implant, which included the collagen scaffold and the printed monolayer of corneal endothelial cells. Over 6 months, the study monitored various parameters such as IOP, corneal health, and structure through high-resolution photography, anterior segment optical coherence tomography (AS-OCT), and confocal microscopy. Comprehensive pathological examinations were performed at the end of the study to understand the biological interactions between the implant and the corneal tissue.
The results were promising and demonstrated the potential of PVEK implants. The sham group exhibited severe corneal edema and stromal scarring, highlighting the necessity of endothelial cells for corneal clarity and function. The scaffold-only group showed significant subepithelial fibrosis and a ~50% detachment rate. The corneas initially achieved a thinner state but often failed, leading to severe edema and a final average central corneal thickness (CCT) of ~460 μm. In contrast, the PVEK group had a markedly lower detachment rate of ~15%, with an average CCT of ~380 μm back to base line thickness of the rabbits. The PVEK corneas remained clear and compact with high endothelial cell counts throughout the study period, indicating successful integration and function of the endothelial cells (Figures 4 and 5).
3. Human results. To date, PVEK has yet to be tested in humans, however we are hoping to perform our first human clinical trial in 2025.
Advantages of PVEK
The PVEK implant offers several advantages over traditional EK
techniques:
1. Consistency and quality. The controlled manufacturing process ensures a consistent product, free from the variability associated with donor tissues. High endothelial cell counts are achieved.
2. Scalability. One donor cornea can produce over 300 PVEK implants, addressing the global shortage of donor tissues.
3. Reduced learning curve. The pre-loaded, ready-to-use design simplifies the surgical process. Furthermore, the graft unfolds easier than a DMEK and more like a DSAEK making it accessible to a broader range of surgeons.
4. Tissue testing for disease transmission prior to grafting. Currently, one to one human cornea transplantation does not involve testing the tissue for pathogens prior to transplantation, putting the patient at risk for possible pathogens. Before the aseptically manufactured PVEK implant is used, comprehensive screening protocols are implemented to detect potential pathogens and contaminants. This involves testing for a wide range of infectious agents, including bacteria, viruses, and fungi, to prevent the transmission of diseases from donor to recipient.
Future Directions
While the current preclinical results are promising, ongoing research and clinical trials are essential to further validate the safety and efficacy of PVEK implants in humans.
Conclusion
The 3D-printed ex vivo-expanded endothelial keratoplasty offers several advantages that make it a promising alternative to traditional EK techniques. The ability to customize the size of the implant, the shape of the marking, and the density of the endothelial cells allows for a tailored approach to each patient’s needs. With the capability to produce over 300 implants from a single human donor, PVEK addresses the critical issue of donor tissue shortage. The rigorous disease testing prior to graft preparation ensures the safety and quality of each implant. The ease of deployment significantly reduces the learning curve compared to DMEK, making it accessible to a broader range of surgeons. However, despite the successful implantation in preclinical and GLP animal studies, it is crucial to validate the safety and efficacy of PVEK in human clinical trials. Continued research and clinical validation are essential to bring this innovative technology to patients. CP
I would like to thank the entire team at Precise Bio (Eitan Livny, Dorin Sade Yazdi, Yishay Hayardeni, Amos Eitan, Lior Rosenberg Belmaker, David Zadok and Michal Marcus) for their dedication and hard work in developing the PVEK technology. Special thanks to my colleagues and collaborators who contributed to the research and clinical studies discussed in this article.
References
1. Oliva MS, Schottman T, Gulati M. Turning the tide of corneal blindness. Indian J Ophthalmol. 2012;60(5):423-427.
2. Droutsas K, Bagikos G, Miltsakakis D, et al. Trends in indications and techniques of corneal transplantation from 1999 through 2015 at a tertiary referral center in Athens, Greece. J Ophthalmol. 2018;2018:9132083.
3. Le R, Yucel N, Khattak S, Yucel YH, Prud'homme GJ, Gupta N. Current indications and surgical approaches to corneal transplants at the University of Toronto: a clinical-pathological study. Can J Ophthalmol. 2017;52(1):74-79.
4. Gain P, Jullienne R, He Z, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134(2):167-173.