The idea of replacing a diseased cornea with an artificial material is not new. The earliest reference goes back to the French ophthalmologist Guillaume Pellier de Quengsy in 1798 who described how such an operation could be performed “without fear.”1 Fast forward to the present day, and the reality of corneal replacement, especially through artificial means, is often met with complexities that de Quengsy could not have foreseen. Those working in the field nowadays would be happy to correct him since these cases are typically very complex and, while it should not be feared, it is certainly not trivial.
Who Needs an Artificial Cornea?
While a century of development in surgical techniques and corneal banking has led to the massive success of corneal transplantation that has surpassed what we can achieve with the current artificial corneas, there are still patients who do not receive corneal grafts. This can either be due to a lack of donor tissue, or that the risk of corneal rejection is so high that it is not a good case for allogenic tissue. These are the patients best suited to artificial corneal implants.
The two leading approaches in artificial corneas are not new and are: the osteo-odonto keratoprosthesis (OOKP) and the Boston KPro (Boston Keratoprosthesis is a not-for-profit entity within Massachusetts Eye and Ear). The OOKP involves using a patient’s tooth as a support structure for the artificial cornea, while the Boston KPro utilizes a clear plastic cylinder that replaces the corneal stroma. Although several newer devices have emerged in recent years, the OOKP and Boston KPro have demonstrated sustainability in clinical applications, largely due to their favorable longitudinal outcomes and lower complication rates.
When Artificial Meets Natural
The main issue in artificial corneas lies in the interface between the synthetic material and the flexible cornea with its viscoelastic properties. The viscoelastic biomechanical properties can be easily seen with Scheimpflug imaging (e.g., with the Corvis device) where the cornea can be seen to have ripples and waves. The interface between the cornea and material is therefore similar to a wave crashing against a sea wall with every movement or blink. This tends to erode the tissue and over time these areas become weakened. As a result, the devices are prone to infection or extrusion. This has always been the greatest challenge in keratoprosthesis.
Endothelium Research
Over the past decades, we have seen major improvements in corneal endothelial grafting — from full thickness corneal grafting to selective replacement of single corneal layers. From penetrating keratoplasty (PK) to Descemet’s stripping automated endothelial keratoplasty (DSAEK) to Descemet’s membrane endothelial keratoplasty (DMEK), the speeds of recovery and clinical outcomes of corneal endothelial keratoplasty have improved so much that they can often return the patient back to 20:20 vision. In the US in 2023, 67% of all corneal transplantation were endothelial keratoplasties.2 The key here is to transplant a high density of corneal endothelial cells that can actively pump fluid out of the stroma, to maintain transparency. This led to major innovations in endothelial cell culture with a view to expanding the donor pool to meet more need,3,4 but these treatments are not cheap and there is still an unmet need that an artificial cornea could serve.5
An Acellular Endothelium
The idea that the endothelium could be replaced by an artificial layer without a cellular pump was unheard of. In 2020, a medical start-up (EyeYon Medical) brought the first artificial corneal endothelium to the operating room. This simple implant is made of a biosynthetic hydrophobic acrylic polymer and challenged our concepts of how to treat corneal edema.
The device does not have a pump function but acts by forming a seal over the posterior stroma preventing the fluid of the anterior chamber from entering. The natural drying effect from the anterior surface over the course of the day is enough to maintain a more transparent cornea. But the implant is a barrier to nutrition too, which limits its diameter so that the peripheral cornea can allow the diffusion of nutrients to the stroma.
Selecting Patients
The device is currently best offered to patients who will not do well with tissue but have a low degree of scarring in the stroma. This typically includes patients with previous failed grafts, multiple rejections and patients with glaucoma tubes who have a poor prognosis for cell loss after endothelial keratoplasty.6,7 Even in patients with a poor visual prognosis, implantation of the artificial graft layer resulted in edema reduction, improved corneal clarity and patients reported a subjective reduction of pain due to reduced bulla formation.
The Surgical Technique
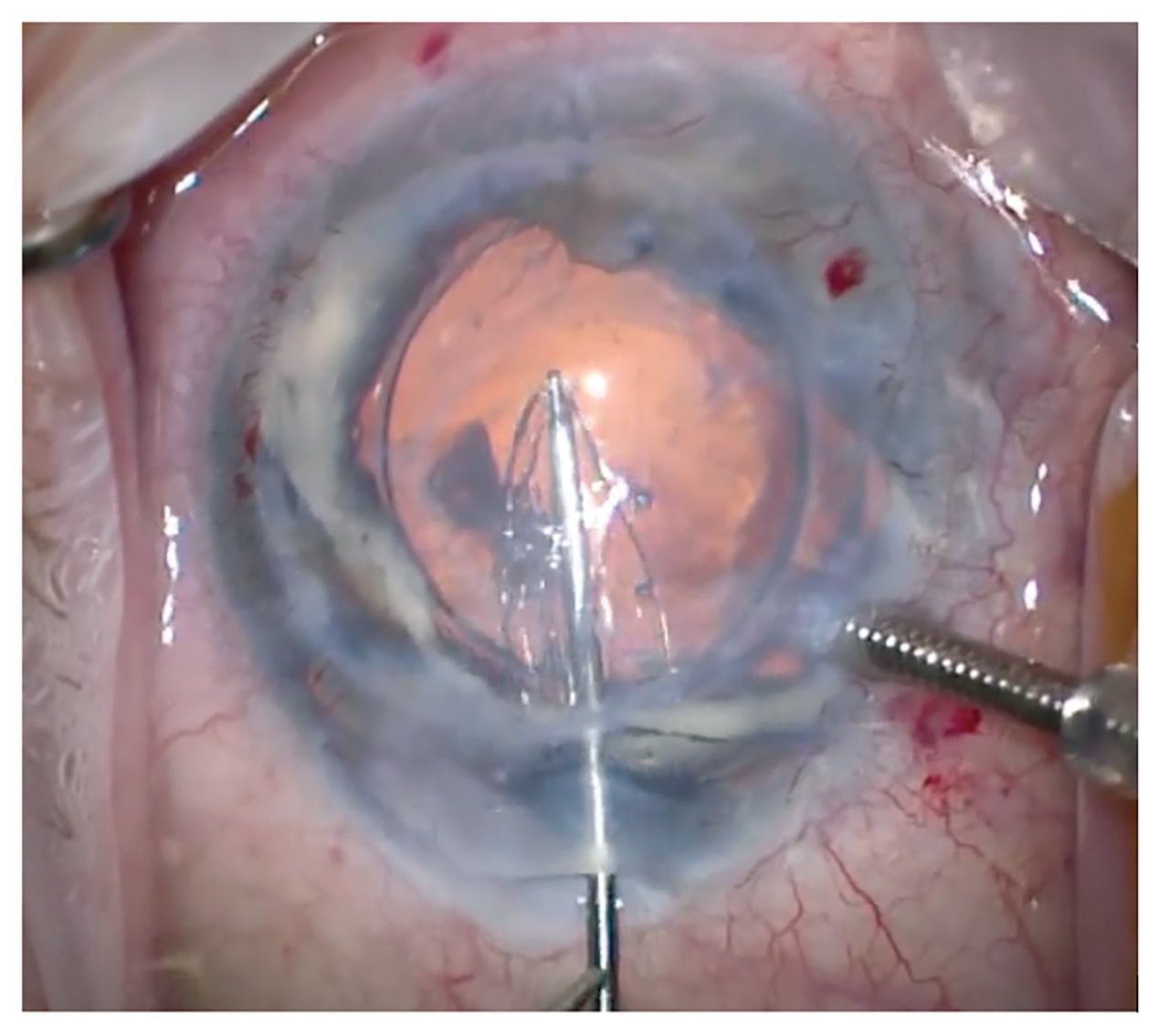
In comparison to using donor tissue, the manipulation of the implant is very simple. The graft can be handled directly with no fear of losing cells (Figure 1). Initially, the grafts had a tendency to detach requiring multiple rebubbles so the surgical technique was adapted to include a longer acting gas tamponade (C3F8) and a fixation suture (Figure 2). I strongly remind the patients not to rub their eyes. The suture can be removed a few weeks later at the slit lamp and this has significantly reduced the detachment rate. Postoperative steroids can also be reduced and stopped, unlike in endothelial keratoplasty.
Future Developments
As it stands, the artificial endothelium is not a challenge to the corneal banking system as it is limited in its diameter and the uncovered areas are prone to swelling and inducing refractive shifts. For wider spread application, more data on the long-term effects of the synthetic grafts are needed to establish the impact of limiting the nutrient flow to the corneal center. In the meantime, these grafts have shown us an entirely new way to approach corneal edema and appear to be an attractive alternative for a group of patients with few good options who are not considered for standard corneal transplantation. CP
References
1. Pellier de Quengsy G, Didot PNF, Méquignon NT, Roullet R. Précis au cours d’operations sur la chirurgie des yeux. Didot, 1789.
2. 2023 Eye Banking Statistical Report. Eye Bank Association of America website. https://restoresight.org/members/publications/statistical-report/
3. Català P, Thuret G, Skottman H, et al. Approaches for corneal endothelium regenerative medicine. Prog Retin Eye Res. 2022;87:100987.
4. Kinoshita S, Koizumi N, Ueno M, et al. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. N Engl J Med. 2018;378(11):995-1003.
5. Kaufman R, Jun AS. Emerging alternatives to keratoplasty for corneal endothelial cell dysfunction. Curr Opin Ophthalmol. 2024;35(5):415-422.
6. Auffarth GU, Son HS, Koch M, et al. Implantation of an artificial endothelial layer for treatment of chronic corneal edema. Cornea. 2021;40(12):1633-1638.
7. Fontana L, di Geronimo N, Cennamo M, Mencucci R, Versura P, Moramarco A. Early outcomes of an artificial endothelial replacement membrane implantation after failed repeat endothelial keratoplasty. Cornea. 2024;43(9):1088-1094.