Corneal blindness is a major global health issue resulting from damage or disease affecting the cornea, the transparent front part of the eye essential for focusing light onto the retina. Corneal blindness disproportionately affects people in low- and middle-income countries with limited access to eye-care services and surgical interventions.1-4 The impact extends beyond the individuals, affecting families and communities while hindering opportunities for education, employment, and quality of life.
Factors contributing to corneal blindness include infections, injuries, genetic disorders, and degenerative conditions. Infections like trachoma and keratitis, trauma from accidents, and congenital disorders can scar or cloud the cornea, significantly impairing vision.
Fortunately, corneal blindness is often treatable through corneal transplantation, replacing damaged or diseased corneal tissue with healthy donor corneas.
Penetrating Keratoplasty
Penetrating keratoplasty (PKP) is the replacement of the central corneal tissue button with a transplant from a donor. This procedure has a failure rate of 20-30%5,6 making repeat transplantations one of the most prevalent indications for the procedure. An additional challenge of this technique is the optical performance following transplantation. A large fraction of patients are left with significant optical distortions which preclude functional vision. Finally, a critical challenge is the shortage of corneal donors and corneal surgeons.
PKP mandates specific training and a significant learning curve to specialize in the procedure and achieve incorporation of the transplant with good optical performance. As a result, many corneally blind patients endure years of waiting for a transplantation, and in some regions, they may never receive the opportunity.
Efforts to address this include raising awareness about organ donation, improving eye banking infrastructure, expanding training programs to include underdeveloped countries and implementing public health strategies to prevent and treat corneal damage. Research into alternative treatments, such as artificial corneas and regenerative medicine, holds promise for expanding options for those affected by corneal blindness. By combining preventive measures, increased access to eye care and advancements in medical interventions, the global community can work towards reducing the burden of corneal blindness and improving vision-related outcomes.
Keratoprostheses
Artificial corneas termed keratoprostheses (KPro), represent a pioneering approach in ophthalmology to address the shortage of donor corneas for transplantation. These devices aim to restore vision by replacing the damaged or diseased natural cornea. While research continues to refine their design and enhance biointegration, artificial corneas offer a promising solution for cases where traditional corneal transplantation may not be feasible or access to transplantation is nonexistent. The evolving field of artificial corneas holds potential in revolutionizing treatment for corneal diseases and improving vision outcomes worldwide.
Early KPros such as the Boston KPro use tissue to bridge the gap between the synthetic lens and the native ocular tissue. Newer approaches aim to eliminate the dependency on tissue for integration, instead utilizing inert and biocompatible polymers. Here is where one of the strengths of the CorNeat KPro lies.
The CorNeat KPro
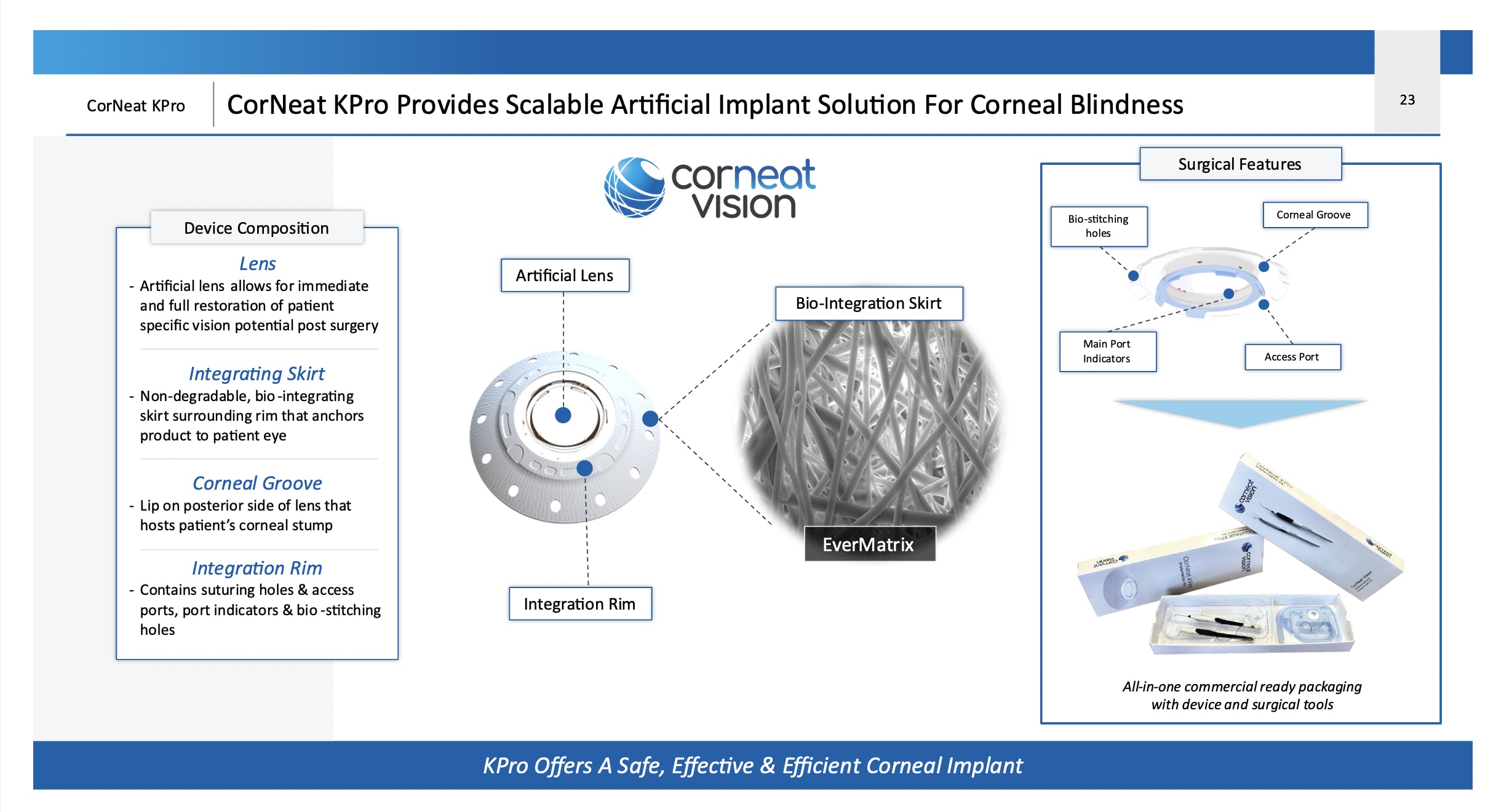
The CorNeat KPro (CorNeat Vision) is an artificial cornea which does not rely on a biological carrier for integration with ocular tissue. Made of a central lens from polymethyl methacrylate (PMMA) delivering 42 D of optical power at the corneal plane, it features a spherical design to compensate for potential decentration. A surrounding porous, non-degradable, polymeric matrix skirt promotes cellular invasion and tissue integration, seamlessly adhering to the eye wall between the conjunctiva and sclera. Previous designs like the Boston and Osteo-Odonto KPros attach to the corneal tissue, a tissue devoid of blood vessels with poor healing capacity, whereas the CorNeat KPro adheres to the eyewall subconjunctivally, an area with abundant blood supply and fibroblasts that promote healing.
Embedding Boston and Osteo-Odonto KPros is very challenging surgically and requires multiple interventions. When setting out to develop the CorNeat KPro device and its implantation procedure the focus was on developing a straightforward, one-step procedure. The development of the procedure went through multiple iterations, first in animal and then in human cadaver eyes. This was followed by the first-in-human (FIH) clinical trial, while feedback from the primary investigators was incorporated throughout.
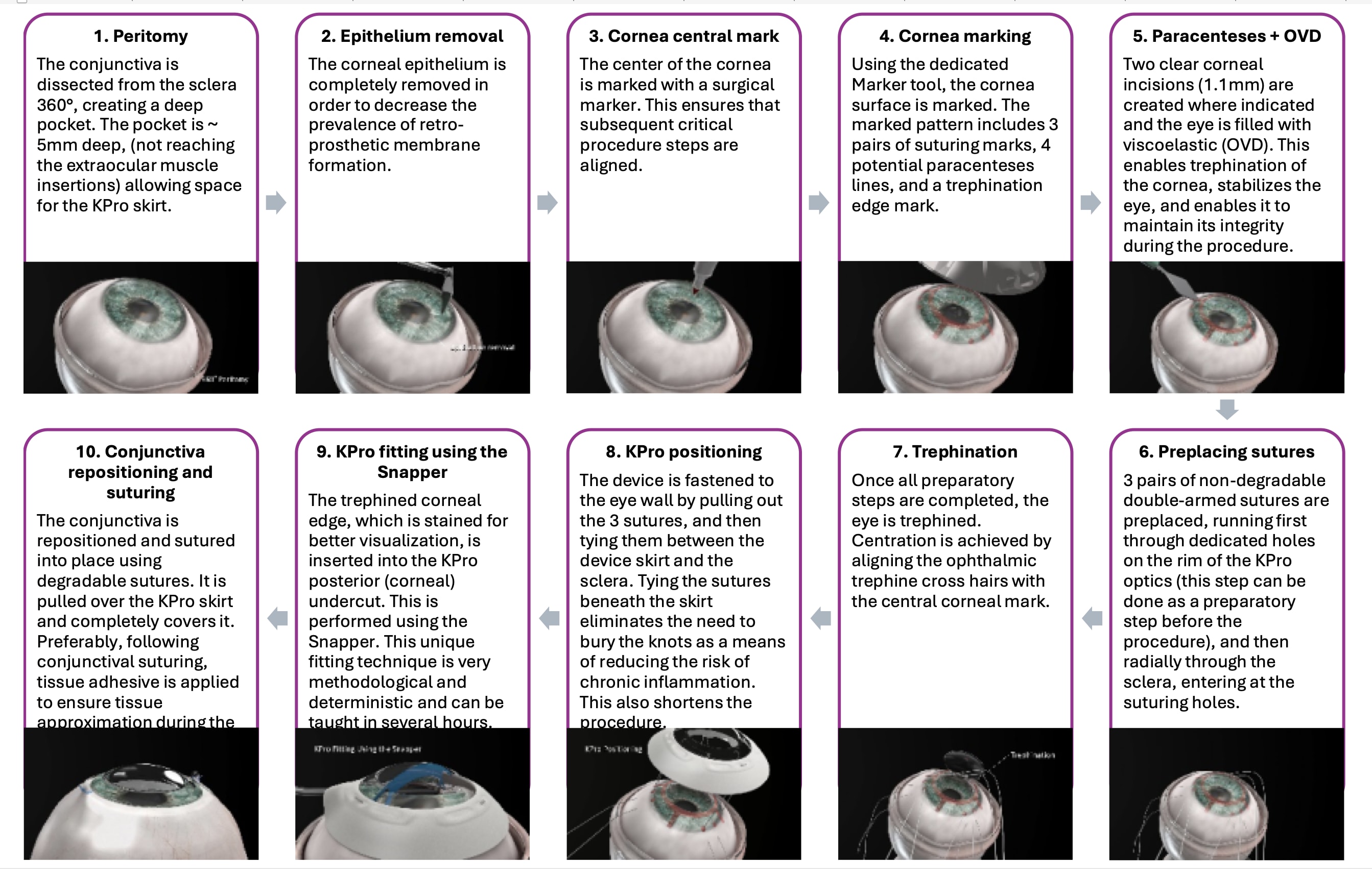
Prior to the FIH trial two tools were developed to aid the surgeon in the implantation — the Marker and Snapper. (Figure 1). The Marker stamps a pattern on the ocular surface at the beginning of the procedure, directing the surgeon where to place paracenteses and sutures. It marks the trephination’s edge to allow better visualization of the remnant cornea to facilitate the complete introduction of the corneal edge into the device’s undercut. The Snapper was developed to assist in the manipulation of the corneal edge into the undercut.
A dedicated training curriculum was developed and includes a 1-hour presentation followed by wet lab practice lasting 2-3 hours per surgeon. Like any procedure, a learning curve was evident in the FIH trial. Contrary to legacy designs, the surgeons mastered the technique relatively easily and quickly.
Many key elements of the design are incorporated into the rim of the PMMA optical member which is fused with the surrounding skirt and covered by it superiorly and inferiorly. The rim of the CorNeat KPro has several important features: (1) dedicated suturing holes in the PMMA, (2) bays in the rim to facilitate future surgical interventions in the anterior chamber, and (3) bio-stitching grooves filled with a porous matrix that encourage tissue growth into the rim, ensuring permanent integration with the eye wall.
The design aims to alleviate the downward pressure on the resident cornea and potential angle closure that ensues. This rim is sutured to the limbal corneosclera with non degradable sutures both physically attaching the device to its intended position and creating a force pulling the angle open.
The CorNeat KPro provides patients with a physiological field of view and excellent visual performance. It offers a valuable treatment option for patients with specific eye conditions, providing renewed independence and quality of life. By replacing the opaque and damaged cornea with a clear and optimal optical lens, patients can achieve their full visual potential, assuming no other pathologies compromise their vision.
When assessing visual performance, visual field is physiological as the optical zone of the CorNeat KPro is 6.5 mm, allowing significantly more light to enter the globe and reach the retina. Best-corrected distance visual acuity (BCDVA), in the CorNeat KPro implantees, demonstrates potential to significantly improve visual acuity in corneally blind patients, as evidenced by significant increases in acuity in nearly all implanted patients at some point during the study, with some achieving 20/20 vision.
First-In-Human Clinical Trial
Following development and extensive biocompatibility testing, the design of the CorNeat KPro was validated in a 6-month good-laboratory-practice rabbit trial,7 leading to ethics committee approvals for the FIH clinical trial.
The surgical procedure was taught to surgeons worldwide through the 1-hour presentation and wet lab training. The FIH trial spanned three countries and continents, involving six surgeons. Patients recruited were terminally blind either following multiple transplantation failures or because they were completely unsuitable for any form of intervention. Lessons learned were incorporated throughout the trial into the protocol and submitted as amendments to various ethics boards, with changes made to the surgical procedure, preoperative, and postoperative care.
The FIH trial included 10 patients who were monitored for 1-2 years. At the trial’s conclusion, five patients retained the device in situ. Four years after the first implantation, three patients continue to have functional vision. Challenges encountered during the trial included surgical difficulties, conjunctival retraction over the skirt in most patients and inflammatory reactions in those with active ocular surface diseases such as Stevens-Johnson syndrome and ocular cicatricial pemphigoid. The device was removed in those cases of OCP, SJS(X2), and in a patient with anti-coagulation treatment that wasn’t stopped prior to surgery resulting in persistent hyphema, and one case of hypotony due to surgical difficulties. These difficulties were addressed later in the trial and resolved. Initially we instructed surgeons to use a 7 mm trephine and eventually we found out a 7.25 mm trephine is the preferred trephination diameter.
As a result of the FIH trial, alterations to the device, surgical procedure, and trial protocol were implemented. The new CorNeat KPro design has a thinner matrix (skirt) with peripheral perforations, allowing direct contact between the conjunctiva and sclera. Additionally, the suturing technique was refined and improved and is detailed in the new protocol. These changes aim to immobilize the conjunctiva soon after the device implantation, achieved by hastening tissue colonization of the matrix while it is immobilized by sutures and creating a tissue bridge at the periphery between the conjunctiva and sclera through the skirt’s peripheral perforations.
To address the challenge encountered during the implantation procedure of fitting of the corneal edge into the posterior undercut of the device, the optical member was redesigned as well as implementing an enlargement of the trephination by 0.25 mm. Regarding protocol alterations, inclusion and exclusion criteria were amended and narrowed, specific guidelines for reporting and tackling adverse events were established, as well as some improvements implemented in the pre and post-op care.
A second human trial is currently recruiting participants at centers in France, Canada, and Israel and ethical approvals are expected in sites in the Netherlands, India, and at a later stage in the United States and China. The second in human trial is designed to support regulatory submissions.
The first implantation in this trial has been performed by Eric Gabison, MD, PhD, the deputy director of the Ophthalmology Department at Rothschild Ophthalmic Foundation and Bichat Hospital, restoring sight to a 71-year-old man in Paris following more than 2 decades of complete blindness. (Figure 4). Only early post-op follow-up is available and so far, the KPro is in situ, no elevation of IOP has been recorded, and his BCDVA is 20/30. CP
References
1. Trachoma Atlas. https://www.trachomaatlas.org.
2. Porth JM, Deiotte E, Dunn M, Bashshur R. A Review of the Literature on the Global Epidemiology of Corneal Blindness. Cornea. 2019;38(12):1602-1609.
3. Qureshi S, Dohlman TH. Penetrating Keratoplasty: Indications and Graft Survival by Geographic Region. Semin Ophthalmol. 2023;38(1):31-43.
4. Wang EY, Kong X, Wolle M, et al. Global Trends in Blindness and Vision Impairment Resulting from Corneal Opacity 1984-2020: A Meta-analysis. Ophthalmology. 2023;130(8):863-871.
5. Qureshi S, Dohlman TH. Penetrating Keratoplasty: Indications and Graft Survival by Geographic Region. Semin Ophthalmol. 2023;38(1):31-43.
6. Borgia A, Romano V, Romano D, et al. Managing Post-Keratoplasty Astigmatism: High-Tech vs. Low-Tech Imaging Techniques for Guiding Suture Manipulation. J Clin Med. 2023;12(10):3462. Published 2023 May 14.
7. Litvin G, Klein I, Litvin Y, Klaiman G, Nyska A. CorNeat KPro: Ocular Implantation Study in Rabbits. Cornea. 2021;40(9):1165-1174.