Optical coherence tomography (OCT), particularly anterior segment OCT, has revolutionized the visualization of biological tissues in vivo.1 Its exceptional ability to generate detailed cross-sectional images has been pivotal in the noninvasive assessment of the cornea and anterior eye segment.2 This advancement is crucial for evaluating patients following refractive surgery, enhancing both pre- and post-operative care.1,2
The continuous evolution of OCT technology coupled with the increasing prevalence of corneal refractive surgeries, has established it as an indispensable tool in modern ophthalmology. However, accurately determining a patient’s past surgical procedures can be challenging, especially in cases where patients are unable to provide a complete history and electronic health records are incomplete or unavailable. In this context, artificial intelligence (AI), particularly deep learning, offers promise by automating the detection and classification of surgical histories from OCT scans.
Our research introduces a deep-learning model developed specifically for OCT applications. This model is adept at automatically identifying various types of keratorefractive surgeries, such as LASIK, photorefractive keratectomy (PRK), and keratorefractive lenticule extraction (KLEx), and further subclassifies them into myopic or hyperopic corrections. By categorizing OCT scans into defined surgical classes, the model significantly decreases IOL miscalculations by identifying corneas with refractive surgeries and recommending appropriate IOL formulas.3 Additionally, it enhances ectasia risk assessments by recognizing post laser vision correction corneas, enabling the application of tailored ectasia detection algorithms as required.4
Study Overview
In this retrospective analysis, we evaluated 14,948 OCT B-scans from 2,278 eyes of 1,166 patients across four international centers. We employed a convolutional neural network utilizing the ResNet18 architecture, which was pretrained on ImageNet to ensure robust feature detection and classification.5,6
Patient OCT B-scans were processed, and data were allocated to training, validation, and testing sets. Our model’s training regimen was enriched with data augmentation techniques and refined through advanced machine learning strategies, including the use of a 1cycle learning rate scheduler and discriminative fine-tuning to optimize performance.7,8
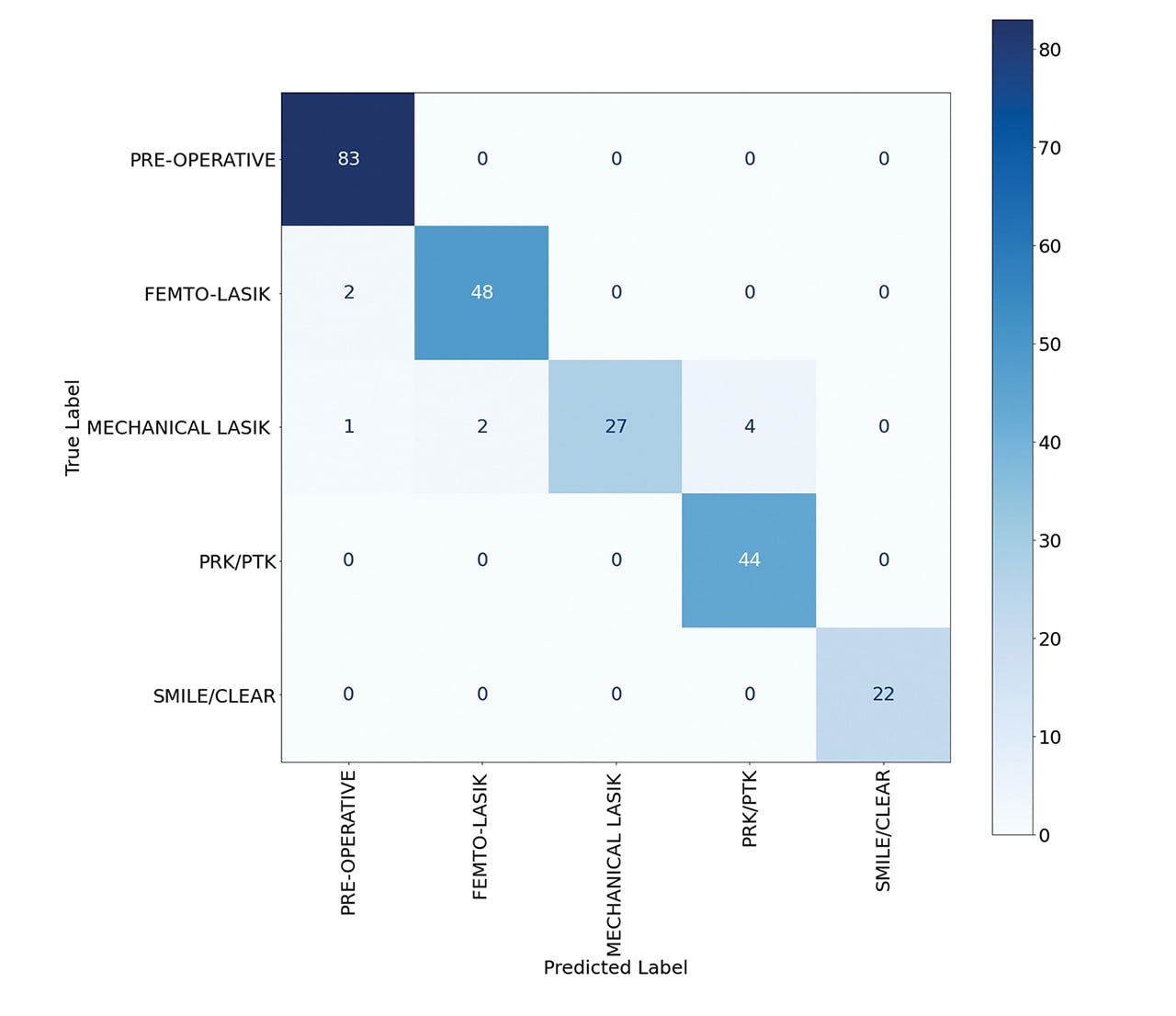
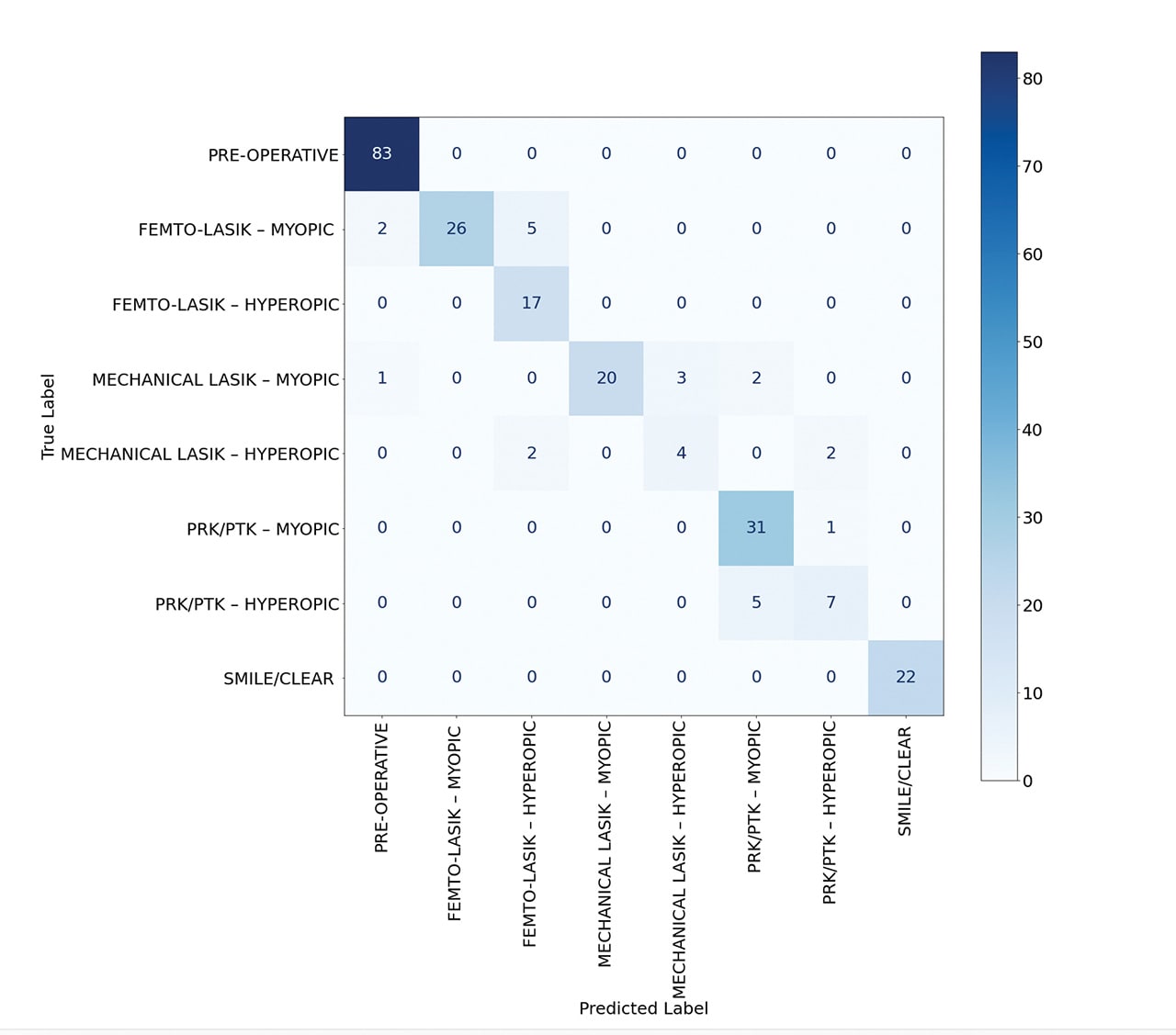
The deep learning model demonstrated remarkable accuracy, achieving 96% in classifying normal, femto-LASIK, mechanical LASIK, PRK, and KLEx surgeries (Figure 1). When the model was trained to identify these surgeries along with their myopic or hyperopic subclasses, it maintained a robust accuracy of 90% (Figure 2).
The model processes multiple 10-mm radial OCT B-scans per patient, sourced from the MS-39 machine (CSO). Therefore, it uses corneal cross-sectional data to derive its classifications. In theory, PRK surgeries are typically identified by the absence of Bowman’s bilaminar membrane,9 while LASIK surgeries are detected by the presence of a flap interface, which varies between femtosecond and mechanical LASIK.10 KLEx surgeries are recognized by their unique, nonpenetrative interface at the peripheral cornea.11 Moreover, myopic or hyperopic corrections can be discerned through an analysis of variations in central and peripheral epithelial thickness.12,13 Given the “black box” nature of deep learning, it is challenging to assert that the model employs conventional decision-making processes. Instead, it may detect subtle indicators that typically elude traditional clinical assessments.
Conclusion
The application of AI in the detection of refractive surgeries from OCT scans is an important milestone in refractive surgery and ophthalmic diagnostics. This study showcases the potential of machine learning to enhance clinical practice by providing ophthalmologists with automated tools for evaluating surgical history, thereby improving patient care. As we continue to amass data and refine AI methodologies, the fusion of AI with ophthalmic imaging promises to set new standards in the field. CP
References
1. Ramos JLB, Li Y, Huang D. Clinical and research applications of anterior segment optical coherence tomography–a review. Clin Experiment Ophthalmol. 2009;37(1):81-89.
2. Ang M, Baskaran M, Werkmeister RM, et al. Anterior segment optical coherence tomography. Prog Retin Eye Res. 2018;66:132-156.
3. Masket S, Masket SE. Simple regression formula for intraocular lens power adjustment in eyes requiring cataract surgery after excimer laser photoablation. J Cataract Refract Surg. 2006;32(3):430-434.
4. Vinciguerra R, Ambrósio R Jr, Elsheikh A, et al. Detection of postlaser vision correction ectasia with a new combined biomechanical index. J Cataract Refract Surg. 2021;47(10):1314-1318.
5. He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. In: Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition. IEEE; 2016:770-778.
6. Russakovsky O, Deng J, Su H, et al. Imagenet large scale visual recognition challenge. Int J Comput Vis. 2015;115:211-252.
7. Smith LN. Cyclical learning rates for training neural networks. In: 2017 IEEE Winter Conference on Applications of Computer Vision (WACV). IEEE; 2017:464-472.
8. Howard J, Ruder S. Universal language model fine-tuning for text classification. ArXiv Prepr ArXiv180106146. Epub 2018.
9. Moilanen JA, Vesaluoma MH, Müller LJ, Tervo TM. Long-term corneal morphology after PRK by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2003;44(3):1064-1069.
10. Xia LK, Yu J, Chai GR, Wang D, Li Y. Comparison of the femtosecond laser and mechanical microkeratome for flap cutting in LASIK. Int J Ophthalmol. 2015;8(4):784-790.
11. Reinstein DZ, Archer TJ, Gobbe M. Accuracy and reproducibility of cap thickness in small incision lenticule extraction. J Refract Surg. 2013;29(12):810-818.
12. Reinstein DZ, Archer TJ, Gobbe M. Change in epithelial thickness profile 24 hours and longitudinally for 1 year after myopic LASIK: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2012;28(3):195-201.
13. Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Epithelial thickness after hyperopic LASIK: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2010;26(8):555-564.
Disclosures: Drs. Assaf and Awwad have financial interest in NeuralVision – FZCO (Dubai, UAE). A full patent has been filed by Drs. Assaf and Awwad pertaining to the methodologies and technologies discussed in this study.