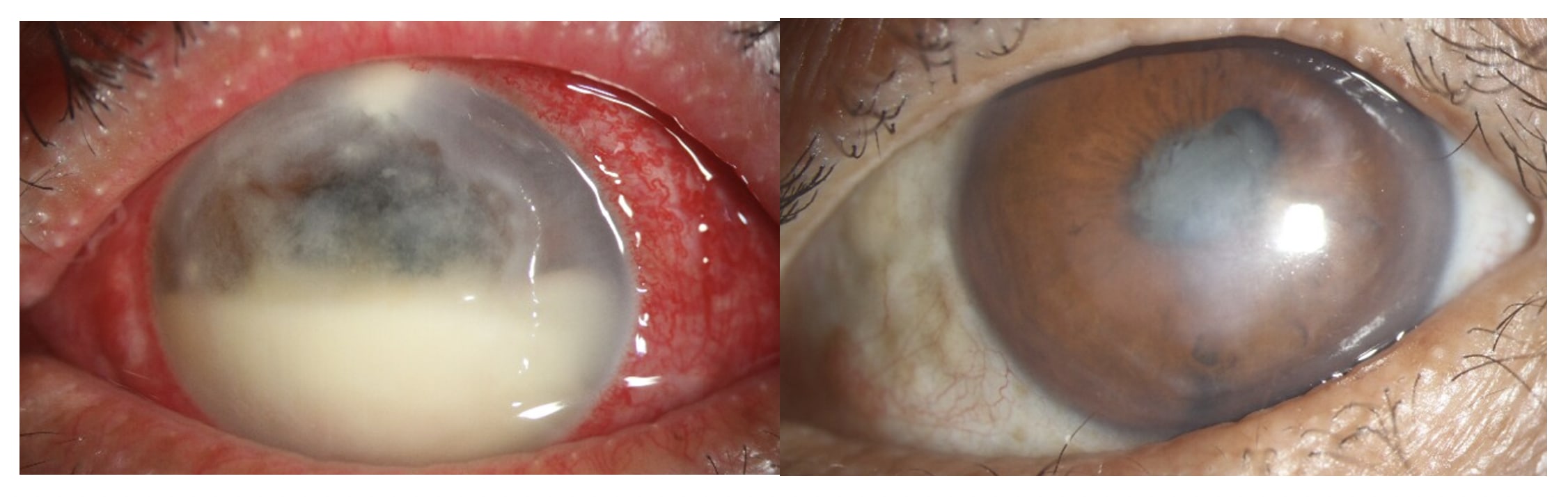
Infectious keratitis (IK), or an infection of the cornea, is a potentially vision-threatening, ophthalmic emergency due to rapid progression and devastating complications. IK is a significant public health issue in the United States,1 as it is a common cause of corneal blindness and the fifth leading cause of overall blindness.2 Back in 2010, the incidence of microbial keratitis in Northern California was estimated to be around 27.6 cases per 100,000/year, for an estimate of 75,000 corneal ulcers annually.3 The main risk factor for corneal infections in the developed world is contact lens use,4 as microorganisms can adhere to the contact lens surface and transfer to a damaged or compromised corneal epithelial layer. Other risk factors are direct trauma, recent corneal surgery, ocular surface disease, chronic use of topical medication, and systemic immunosuppression.
IK can be caused by a variety of microorganisms, including bacteria, fungi, viruses, and parasites. Each type of infection presents distinct clinical features and requires specific management strategies. Prompt diagnosis of the causative microorganism and early treatment with the appropriate topical/systemic antimicrobial medication is crucial for effective treatment. Advances in topical antimicrobial therapy, especially the development of fourth-generation fluoroquinolones and fortified antibiotics, have enabled effective broad-spectrum treatment with good tissue penetration. Patients with atypical bacterial infections caused by Mycobacteria and Nocardia spp. have poorer outcomes. Moreover, the recent pan resistant Pseudomonas aeruginosa outbreak highlights the growing threat of antimicrobial resistance.
Likewise, corneal infections caused by fungal species continue to pose significant challenges in management. The current treatment guidelines for fungal infections come from the Mycotic Ulcer Treatment Trial5 which compared the efficacy of topical natamycin and voriconazole to treat Fusarium infections. Natamycin demonstrated superiority, while topical voriconazole or amphotericin B showed superiority for Candida infections. Parasitic ulcers, particularly Acanthamoeba keratitis, represent the greatest therapeutic challenge. While polyhexamethylene biguanide and chlorhexidine are often employed in management, their efficacy can be limited due to the organism’s ability to form resistant cysts and the often delayed diagnosis.
Despite existing treatment guidelines and advancements in antimicrobial therapies, the management of severe cases of infectious keratitis remains a complex challenge. The emergence of antibiotic-resistant organisms coupled with limited access to appropriate treatments has led to a significant increase in severe ocular complications. These complications, including corneal melt, iris synechiae, secondary ocular hypertension, optic neuropathy, corneal perforation, endophthalmitis, and hemorrhagic choroidal detachment, can ultimately result in permanent vision loss or even enucleation. To prevent corneal perforation or further infection spread to the sclera or the interior of the eye, therapeutic penetrating keratoplasty or therapeutic lamellar keratoplasty may be necessary. However, corneal grafts are at increased risk of failure in the presence of active corneal inflammation. Given the potential for severe visual impairment and limited treatment options, alternative therapeutic approaches are essential for managing severe, progressive corneal infections.
Photodynamic Antimicrobial Therapy
During the last 20 years, corneal collagen crosslinking (CXL), traditionally used for corneal ectasia, has been explored in the management of infectious keratitis. This procedure involves the application of a photosensitizer to the cornea which is activated with light of a specific wavelength. In CXL, riboflavin (vitamin B2) is activated with ultraviolet-A (UV-A) light to generate reactive oxygen species (ROS). The ROS interact with the corneal collagen fibers to create new crosslinks in adjacent fibrils and strengthen the cornea. The same methodology was applied to cases of IK as a way to halt the corneal melting that occurs secondary to infections. Additionally, the ROS species also can kill the microorganisms that are causing the IK. When applying riboflavin/UVA for antimicrobial purposes, this procedure is known as photo-activated chromophore for keratitis corneal crosslinking (PACK-CXL). PACK-CXL was found to have some success against bacterial strains, but less efficacy against fungal infections. This led to the exploration of a more effective photosensitizer, Rose
Bengal (RB).
Rose Bengal
Rose Bengal is routinely used in ophthalmology clinics to stain for corneal or conjunctival epithelial defects and degeneration. The main constituents of RB are iodine derivatives of di- and tetrachlorofluorescein. Like riboflavin, when RB is activated by light at a specific wavelength (520 nm, green light) ROS are generated, but a much greater quantity. These highly reactive molecules attack essential cell components, ultimately leading to cell death of the microorganisms.
Over the past decade, RB Photodynamic Antimicrobial Therapy (RB-PDAT) has been explored as a potential treatment for infectious keratitis. The in vitro inhibitory effect against many microorganisms has been tested extensively, with over 20 peer reviewed publications. Microbial inhibition has been observed in the following species: (bacteria) Staphylococcus aureus, Pseudomonas aeruginosa, and Nocardia spp.; (Fungi) Candidaspp.,Fusarium spp.,Paecilomyces spp., Pseudallescheria boydii, Curvularia lunata; and (Parasites) Acanthamoeba spp.6-11 In vivo safety studies have confirmed that despite inducing damage to the invading, infectious cells, RB-PDAT is safe for the eye; showing the preservation of the corneal endothelium, limbal stem cell niche, iris, keratocytes as well as the photoreceptors in the retina.
Based on the in vitro efficacy and in vivo safety data, this technology was applied to patients with severe corneal infections. Bascom Palmer Eye Institute (BPEI) has treated over 130 patients and the outcomes have been promising. BPEI has emerged as a referral center for this therapy, attracting patients from across Florida, the United States, and even Latin America and the Caribbean. In 2019, a pilot clinical study involving 18 patients with severe infectious keratitis was conducted to translate preclinical findings into clinical practice. While the study demonstrated a 72% success rate, the limited sample size and microbial diversity precluded definitive conclusions.6 A larger dataset would enable a more comprehensive evaluation of the clinical efficacy of RB-PDAT.
Additionally, there is currently an National Institutes of Health-sponsored clinical trial to assess the efficacy of RB-PDAT to treat fungal and Acanthamoeba keratitis: The Rose Bengal electromagnetic activation with green light for infection reduction12 in a double-masked, prospective, randomized, clinical trial. Two international sites are conducting the study: Madurai, India, and Sao Paulo, Brazil. Notably, Aravind Eye Hospital in Madurai, India has also reported treating over 100 patients with this procedure. Patients with smear or culture positive fungal or Acanthamoeba keratitis, or smear and culture negative corneal ulcers with moderate to severe vision loss (Snellen visual acuity of 20/40 or worse) are eligible for inclusion. The clinical treatment involves the application of 0.1% RB solution in sodium chloride applied to the cornea for a 30-minute soak. After the soak, the cornea is rinsed and the 6 mW green LED light is applied for 15 minutes. This study will provide critical insights into the future viability of RB-PDAT for treatment.
Moreover, the University of Miami has entered into a licensing agreement with Provectus Biopharmaceuticals, a company that specializes in pharmaceutical-grade RB. This represents a strategic partnership aimed at accelerating the development and commercialization of PDAT for the treatment of infectious keratitis, benefiting patients worldwide through an effective partnership that combines cutting-edge research with industry expertise.13
Infectious keratitis is a serious condition with significant implications for vision. RB-PDAT has demonstrated potential as a valuable adjunct therapy for refractory infectious keratitis. The efficacy and safety of RB-PDAT will be further defined upon completion of ongoing clinical trials. CP
References
1. Collier SA, Gronostaj MP, MacGurn AK, et al; Centers for Disease Control and Prevention (CDC). Estimated burden of keratitis--United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(45):1027-1030.
2. Ung L, Acharya NR, Agarwal T, et al. Infectious corneal ulceration: a proposal for neglected tropical disease status. Bull World Health Organ. 2019;97(12):854-856.
3. Jeng BH, Gritz DC, Kumar AB, et al. Epidemiology of ulcerative keratitis in Northern California. Arch Ophthalmol. 2010;128(8):1022-1028.
4. Ung L, Bispo PJM, Shanbhag SS, Gilmore MS, Chodosh J. The persistent dilemma of microbial keratitis: Global burden, diagnosis, and antimicrobial resistance. Surv Ophthalmol. 2019;64(3):255-271.
5. Venkatesh Prajna N, Krishnan T, Mascarenhas J, et al; Mycotic Ulcer Treatment Trial Group. The Mycotic Ulcer Treatment Trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013;131(4):422-429.
6. Naranjo A, Arboleda A, Martinez JD, et al. Rose Bengal photodynamic antimicrobial therapy (RB-PDAT) for patients with progressive infectious keratitis: a pilot clinical study. Am J Ophthalmol. 2019;208:387-396.
7. Arboleda A, Durkee H, Miller D, et al. Variations in irradiation energy and rose bengal concentration for photodynamic antimicrobial therapy of fungal keratitis isolates. Lasers Med Sci. 2024;39(1):72.
8. Amescua G, Arboleda A, Nikpoor N, et al. Rose Bengal photodynamic antimicrobial therapy: a novel treatment for resistant Fusarium keratitis. Cornea. 2017;36(9):1141-1144.
9. Arboleda A, Miller D, Cabot F, et al. Assessment of rose bengal versus riboflavin photodynamic therapy for inhibition of fungal keratitis isolates. Am J Ophthalmol. 2014;158(1):64-70.
10. Halili F, Arboleda A, Durkee H, et al. Rose Bengal- and riboflavin-mediated photodynamic therapy to inhibit methicillin-Resistant Staphylococcus aureus keratitis isolates. Am J Ophthalmol. 2016;166:194-202.
11. Durkee H, Arboleda A, Aguilar MC, et al. Rose bengal photodynamic antimicrobial therapy to inhibit Pseudomonas aeruginosa keratitis isolates. Lasers Med Sci. 2020;35(4):861-866.
12. Prajna V, Prajna L, Sharma S, et al. A double-masked, sham-controlled trial of rose bengal photodynamic therapy for the treatment of fungal and acanthameoba keratitis: Rose Bengal Electromagnetic Activation with Green Light for Infection Reduction (REAGIR) Study. Res Sq [Preprint]. 2024 Jul 2:rs.3.rs-4165312.
13. Provectus Biopharmaceuticals announces exclusive worldwide license agreement with University of Miami for photodynamic antimicrobial treatment of different eye infections with rose Bengal sodium. Provectus Biopharmaceuticals website. Accessed August 18, 2024. https://www.provectusbio.com/news/press-releases/license-agreement-with-university-of-miami-photodynamic-antimicrobial-treatment-eye-infections-rose-bengal-sodium/.