Pre-Descemet’s endothelial keratoplasty (PDEK) involves the transplantation of Descemet’s membrane with endothelium and pre-Descemet’s layer).1 It is a selective transplantation of the endothelium-Descemet’s membrane complex with pre-Descemet’s layer. Introduced by Agarwal et al in 2013 by incorporating the novel Dua’s layer, PDEK has been performed as a viable endothelial keratoplasty for various indications in eyes which have endothelial decompensation.2 The initial results in the pastdecade have been promising showing good functional and anatomical outcomes.1,3,4,5,6
Indications and preoperative assessment
The common indications for PDEK are Fuchs endothelial corneal dystrophy, pseudophakic bullous keratopathy, posttraumatic corneal decompensation, and aphakic bullous keratopathy. The preliminary preoperative assessment includes visual acuity, slit lamp biomicroscopy, clinical digital photography, IOP, and fundus assessment. Corneal analysis includes pachymetry, anterior-segment optical coherence tomography (OCT), specular microscopy (endothelial density),and topography wherever possible. Endothelial cell analysis and topography may be difficult due to diffuse edema and scarring in few cases. Preoperative retinal evaluation can be done by ultrasound B scan in eyes with dense scarring which obscures visibility. Comorbidities like dry eye and adnexal problems have to be addressed and managed in selected cases.
PDEK graft harvesting from donor eye
A corneoscleral disc with an approximately 2-mm scleral rim is dissected from the whole globe or obtained from an eye bank.1 A 30-gauge needle attached to a 5-mL syringe is inserted from the limbus into the mid peripheral stroma (Figure 1A). Air is then injected into the donor stroma until a type 1 big bubble is formed (Figure 1B). When a PDEK graft of precise dimension is needed, eg., for a failed penetrating keratoplasty,the big bubble is deflated and a trephine is used to cut the graft to the desired size (Figure 1C). The bubble wall is penetrated at the extreme periphery with a side-port blade and trypan blue is injected to stain the graft. The trypan blue is then washed out and the graft is gently cut with a pair of Vannas scissors held horizontally. (Figure 2). The harvested PDEK graft is placed in a storage medium in a bowl on the operating table (Figure 3).
Recipient bed preparation
Under peribulbar anesthesia, with sterile precautions, the patient’s eye is opened with a wide speculum. Epithelial debridement is done to remove the hypertrophic epithelium using an iris repositor or rod. This improves the intraoperative visualization. Placing a trocar anterior chamber maintainer (TACM) inside the eye is important for anterior chamber stability, and air is infused into the AC using an air pump mechanism.7 The recipient Descemet’s membrane is stripped off from the host cornea using a reverse Sinskey hook. An inferior peripheral iridectomy is created. The PDEK graft is then inserted into the AC with an improvised injector. The donor tissue rolls as a scroll. It is then unscrolled with fluid and air. The graft is finally attached to the recipient bed by injecting an air bubble beneath it, with further air being injected to fill the AC. Once the graft is centered and adhered, the trocar is removed. Corneal stab incisions are closed with 10-0 monofilament nylon sutures.
Postoperative treatment
Postoperatively, PDEK patients are given topical steroid and moxifloxacin combination eye drops for and initial 1 month. Topical steroids are tapered after 1 month and stopped by 6 months period. Topical lubrication with hydroxyl propylmethyl cellulose drops is often required for initial 1 month postoperatively.
PDEK with glued IOL
PDEK can be combined with various other surgical procedures. In eyes with deficient capsules, it is combined with glued trans-scleral — fixated IOL (Figure 4). Under peribulbar anesthesia, epithelial debridement is performed for better visualization. In this combined procedure, glued IOL is preceded before PDEK technique.7 After inserting a TACM, two partial thickness limbal-based scleral flaps are made 180° opposite to each other.8 A sclerotomy is made with a 23-G needle approximately 1.5 mm from the limbus beneath the scleral flaps, and vitrectomy is performed with the vitreous cutter introduced through the sclerotomy sites.9 A 2.8-mm clear corneal tunnel is performed, a three-piece foldable IOL is loaded into the cartridge, and is introduced into the eye. Anend opening glued IOL forceps is introduced from the sclerotomy site, and the tip of the leading haptic is grasped followed by its externalization. The trailing haptic is also externalized using a handshake technique through the opposite sclerotomy.10 The haptic then is tucked into anintrascleral Scharioth tunnel made from the edge of the scleral flaps. Scleral flaps are then closed with fibrin glue. Once the IOL is secured, PDEK can be followed during he same procedure using the air pump-assisted and endoilluminator-assisted PDEK techniques (described later). The AC is finally formed with air.
PDEK with single-pass four-throw pupilloplasty
Single-pass four-throw (SFT) pupilloplasty is an easy alternative pupilloplasty method which has shown good functional results.11 Maintaining an iris-IOL diaphragm is crucial in PDEK, and for that matter, in any endothelial keratoplasty to prevent air escape and AC collapse.12 This also prevents the graft from falling into the posterior segment and reduces the risk of postoperative angle closure glaucoma or fixed dilated pupil (Urrets-Zavalia syndrome).13
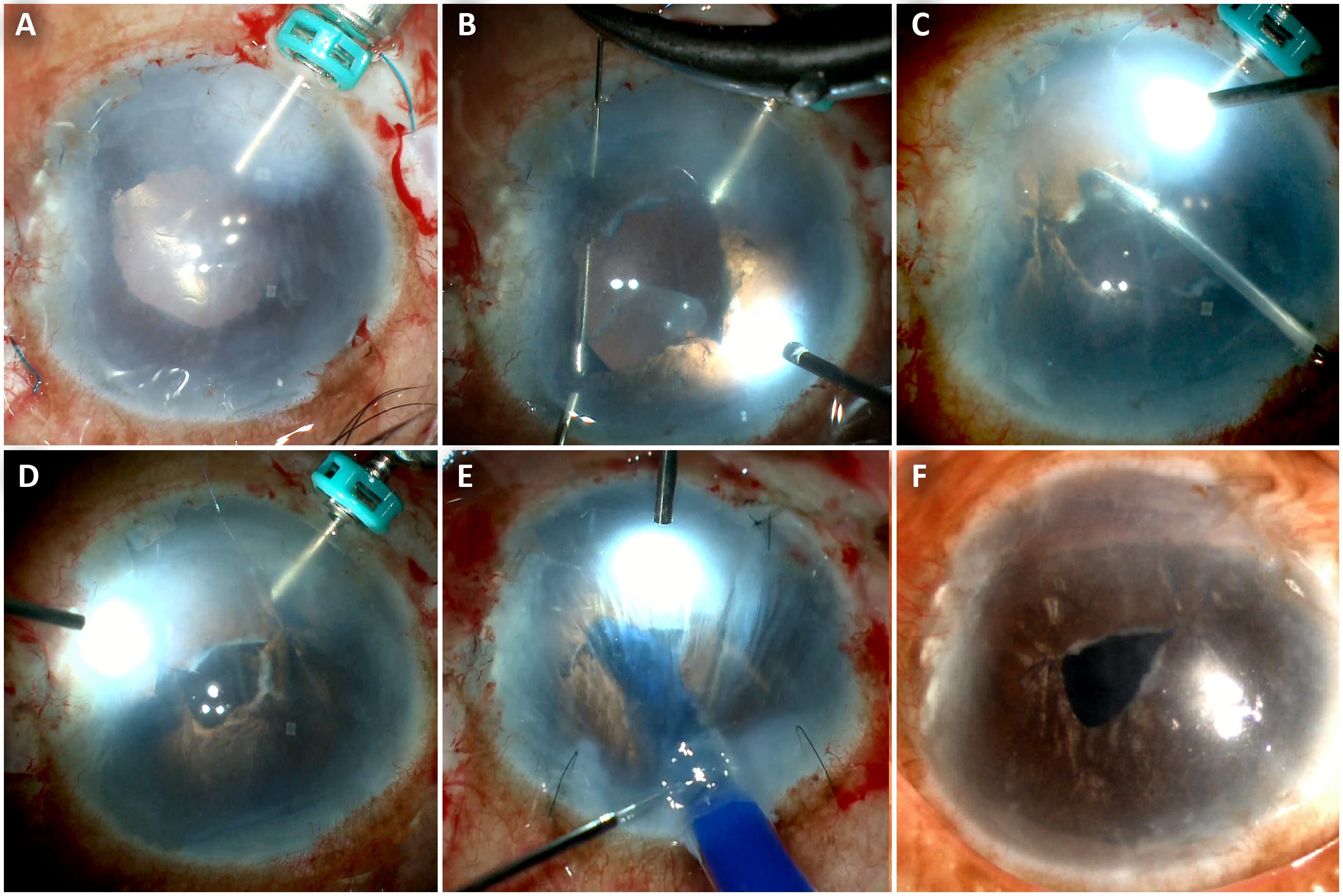
In SFT, an end-opening forceps is initially introduced from the paracentesis incision and used to grasp the distal end of the iris tissue (Figure 5). The tip of a 9-0 polypropelene needle is passed through this iris leaflet. A 26-G needle then passes through the proximal iris leaflet. The needle of the Prolene suture is docked into the barrel of the 26-G needle that is then eventually pulled out from the AC, and this facilitates the exit of the needle from the AC.11 A Sinskey hook or end-opening forceps is passed from the paracentesis incision, and pulling the suture end from the exit site creates a loop when withdrawn from the eye. The suture end is passed through the loop four times, taking care to always pass it in the same direction. In this way, four throws are taken, and intertwining of the sutures creates a helical configuration. Both suture ends are pulled from either side, and this leads to sliding of the helicalloop inside the AC, thereby approximating the iris defect; the suture ends are cut with microscissors.
E-PDEK or endoilluminator-assisted PDEK
This technique was described by one of the authors (Soosan Jacob, MS, FRCS, DNB). Graft apposition is aided by intraoperative endoilluminator.14 Proper alignment of the graft is done using an endo-illuminator (E-PDEK) focused on the surface of the hazy cornea. The visibility is enhanced by good illumination which helps in depth assessment and the scroll edges can be nicely delineated (Figure 5). The PDEK graft is then opposed to the host cornea by pneumatic adhesion using air beneath the scroll.
Air-pump assisted PDEK
Also described by Dr. Jacob, pressurized air infusion is provided through a TACM connected to the fluid-air exchange system of a posterior vitrectomy machine during surgery.15 Pressurized air infusion within the AC helps to perform descemetorhexis, prevents bleeding during peripheral iridectomy and synechiolysis, and prevents oozing of blood into the AC from peripheral corneal neovascularization. It also aids in precise graft manipulation, centration, edge unfolding, and graft adhesion. It prevents AC depth fluctuations during intracameral maneuvers and increases intra-operative graft adherence, thereby also decreasing intraoperative as well as postoperative incidence of graft detachments.16
Triple procedure
Combining PDEK with glued IOL and pupilloplasty technique is noted as a triple procedure. The triple procedure of glued IOL allows secondary IOL fixation along with single-pass four-throw pupilloplasty that prevents the passage of air into the vitreous cavity and maintains air tamponade. This is followed by pre-Descemet's endothelial keratoplasty that replaces the dysfunctional host endothelial layer, thereby regaining new functionality.9 This triple procedure is often required in eyes with decentered or malpositioned IOLs causing secondary endothelial decompensation and irregular pupil.12 It can also be combined with silicone oil removal in vitrectomized eyes (Figure 6). Pinhole pupilloplastyis a modification of SFT where the pupil is reduced to a size of pinhole, thereby utilizing the pinhole optics for better visual outcome.17 This is performed in eyes with coexisting corneal scars or higher-order aberrations.
Functional results with PDEK
PDEK in failed previous therapeutic penetrating keratoplasty
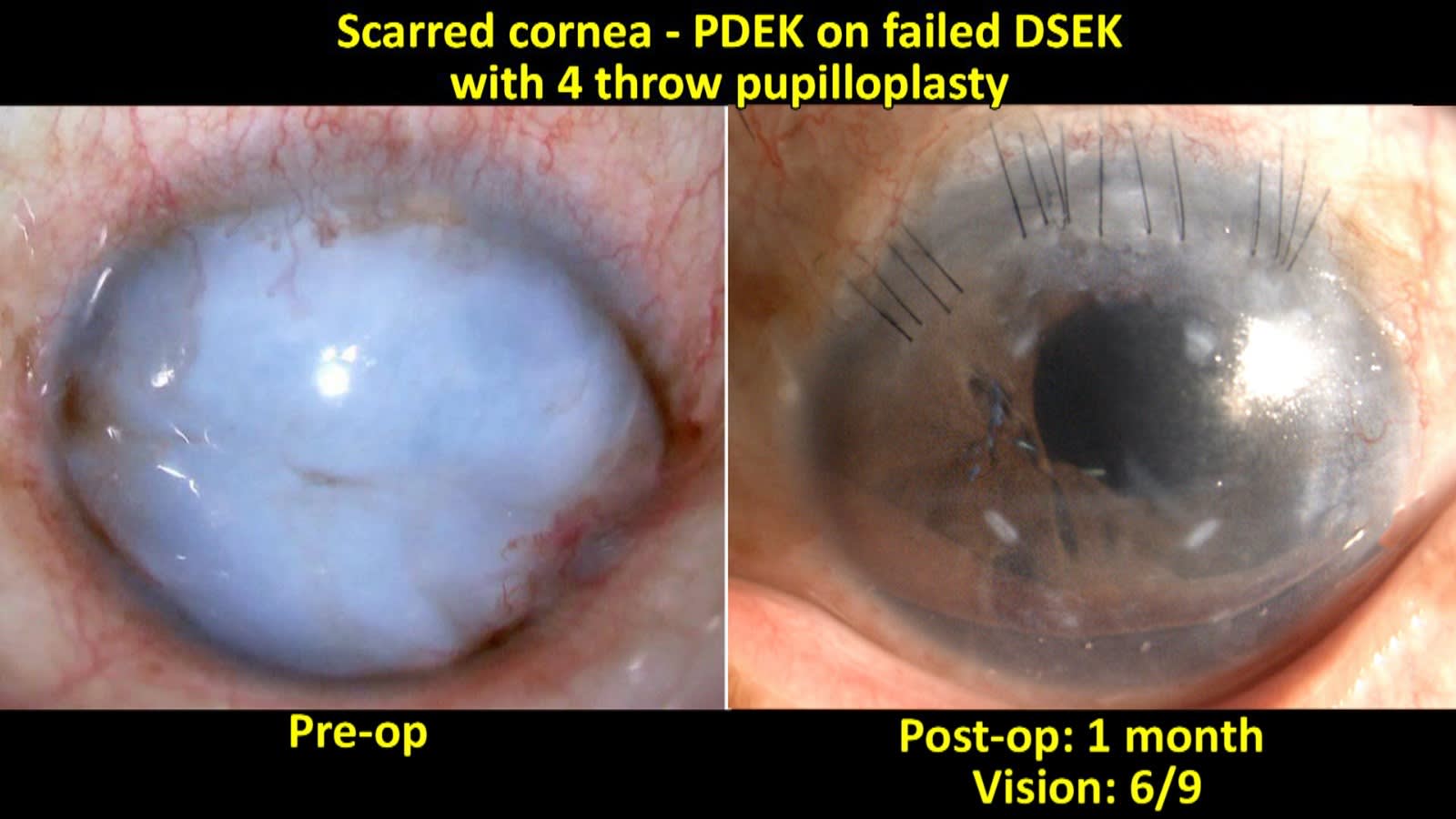
In an interventional study on PDEK in failed therapeutic penetrating keratoplasty (TPK) scenario, we have noted good functional improvement.18 There was significant visual acuity improvement and the percentage of endothelial cell density loss was 28.2% ± 10.6%. Rebubbling rate was minimal. Thus PDEK can be considered as a viable alternative in failed TPK eyes, failed Descemet’s membrane endothelial keratoplasty (DMEK) or Descemet’s stripping endothelial keratoplasty (DSEK) eyes for visual improvement (Figure 7).
PDEK densitometry analysis
In this prospective study, 35 eyes (21 PDEK eyes and 14 control eyes) were evaluated.6 The mean total densitometry values in the anterior, central, and posterior corneas were 40.7 ± 7.6 GSU, 25.3 ± 4.8 GSU, and 20.9 ± 3.4 GSU, respectively. In eyes with BCVA 20/20, there was no significant difference between PDEK and controleyes in the total corneal densitometry in the entire depth of the cornea (P = 0.662).
Young donor PDEK
An advantage of PDEK is the graft availability from young donors. A study by Agarwal et al, reported that PDEK using young donor grafts resulted in significantly improved visual acuity to a functional level, with the clearance of subepithelial fibrosis and anterior stromal scar.4 In another report on an infant donor (younger than 1 year), the mean percentage loss of endothelial cells at 6 months was observed to be 27 ± 2%.3 No incidence of tissue loss during graft preparation, graft dislocation, or graft failure was reported. The mean graft thickness as measured with OCT on the first postoperative day was 35 ± 3 μm. As younger grafts are used, there is better endothelial function and the loss may be compensated by more efficient cell function.
PDEK OCT analysis
In the aspectral-domain OCT study by us, the PDEK graft thickness was noted to be thicker than DMEK grafts.6 The mean graft thickness of PDEK tissue on day 1 was 37.3±3.5µm (range 32- 44µm). There was a significant difference in the graft thickness over the time period (P=.000). There was no significant difference between the central and peripheral graft thickness. On OCT, there was minimal reversible or no interface opacification in the postoperative period. Because of the very slightly thicker graft size as compared to DMEK, PDEK shows good intraoperative stability and graft adhesion.19 The minimally thicker nature of the PDEK graft helps to reduce the endothelial loss and inadvertent graft damages from unnecessary maneuvering intraoperatively. There is no increased risk of graft dislocation due to thinness as compared to DSEK grafts.20
Conclusion
PDEK has the advantage of thinner grafts, which can aid in easy intraoperative manipulation and at the same time better postoperative adherence. The PDEK graft has advantages over other endothelial keratoplasties, like usability of young donor, less manipulation-induced endothelial loss, decreased postoperative refractive shift, no drawbacks due to thicker grafts, and early anatomical and visual rehabilitation. The preparation of PDEK lenticule is not difficult in the current clinical practice, as pneumatic dissection can be performed by all corneal surgeons. Simple technique and an easy learning curve makes the technique popular and functionally useful for many patients. CP
References
1. Agarwal A, Dua HS, Narang P, et al. Pre-Descemet’s endothelial keratoplasty (PDEK). Br J Ophthalmol. 2014;98(9):1181-1185.
2. Dua HS, Faraj LA, Said DG, Gray T, Lowe J. Human corneal anatomy redefined: a novel pre-Descemet’s layer (Dua’s layer). 2013;120(9):1778-1785.
3. Agarwal A, Agarwal A, Narang P, Kumar DA, Jacob S. Pre-Descemet endothelial keratoplasty within fant donor corneas: a prospective analysis. Cornea. 2015;34(8):859-865.
4. Agarwal A, Narang P, Kumar DA, Agarwal A. Young donor-graft assisted endothelial keratoplasty (PDEK/DMEK) with epithelial debridement for chronic pseudophakic bullous keratopathy. Can J Ophthalmol. 2017;52(5):519-526.
5. Kumar DA, Dua HS, Agarwal A, Jacob S. Postoperative spectral-domain optical coherence tomography evaluation of pre-Descemet endothelial keratoplasty grafts. J Cataract Refract Surg. 2015;41(7):1535-1536.
6. Kumar DA, Agarwal A, Jaganathasai N. Densitometry analysis of corneal backscatter after pre-Descemet endothelial keratoplasty for pseudophakic bullous keratopathy. Cornea. 2020;39(1):30-38.
7. Narang P, Agarwal A. Triple procedure for pseudophakic bullous keratopathy in complicated cataract surgery: glued IOL with single-pass four-throw pupilloplasty with pre-Descemet's endothelial keratoplasty. J Cataract Refract Surg. 2019;45(4):398-403.
8. Agarwal A, Narang P, Kumar DA, Agarwal A. Trocar anterior chamber maintainer: improvised infusion technique. J Cataract Refract Surg. 2016;42(2):185-189.
9. Agarwal A, Kumar DA, Jacob S, Baid C, Agarwal A, Srinavasan S. Fibrin glue-assisted sutureless posterior chamber intraocular lens implantation in eyes with deficient posterior capsules. J Cataract Refract Surg. 2008;34(9):1433-1438.
10. Agarwal A, Jacob S, Kumar DA, Agarwal A, Narasimhan S, Agarwal A. Handshake technique for glued intrascleral haptic fixation of a posterior chamber intraocular lens. J Cataract Refract Surg. 2013;39(3):317-322.
11. Narang P, Agarwal A. Single-pass four-throw technique for pupilloplasty. Eur J Ophthalmol. 2017;27(4):506-508.
12. Narang P, Agarwal A, Dua HS, Kumar DA, Jacob S, Agarwal A. Glued intrascleral fixation of intraocular lens with pupilloplasty and pre-Descemet endothelial keratoplasty: atripleprocedure. Cornea. 2015;34(12):1627-1631.
13. Narang P, Agarwal A, Ashok Kumar D. Single-pass four-throw pupilloplasty for Urrets-Zavalia syndrome. Eur J Ophthalmol. 2018;28(5):552-558.
14. Jacob S, Agarwal A, Agarwal A,Narasimhan S, Ashok Kumar D, Sivagnanam S. Endoilluminator-assisted transcorneal illumination for Descemet membrane endothelial keratoplasty: enhanced intraoperative visualization of the graft in corneal decompensation secondary to pseudophakic bullous keratopathy. J Cataract Refract Surg. 2014;40(8):1332-1336.
15. Jacob S. Use of pressurized air infusion for pre Descemet's endothelial keratoplasty (PDEK) - the air pump assisted PDEK technique. Open Ophthalmol J. 2018;12:175-180.
16. Jacob S, Narasimhan S, Agarwal A, Agarwal A, SaijimolAI. Air pump-assisted graft centration, graft edge unfolding, and graft uncreasing in young donor graft pre-Descemet endothelial keratoplasty. Cornea. 2017;36(8):1009-1013.
17. Kumar DA, Agarwal A, Ravichandran S. Pre-Descemet's endothelial keratoplasty with glued intraocular lens implantation with pinhole pupilloplasty in a case of ocular comorbidity in achromatopsia. Taiwan J Ophthalmol. 2024;14(1):112-116.
18. Narang P, Ashok Kumar D, Narang R, Agarwal A. Outcomes of pre-Descemet endothelial keratoplasty for failed therapeutic penetrating keratoplasty. Cornea. 2023 Sep 12. [Online ahead of print]
19. Guerra FP, Anshu A, Price MO, Giebel AW, Price FW. Descemet’s membrane endothelial keratoplasty: prospective study of 1-year visual outcomes, graft survival, and endothelial cell loss. Ophthalmology. 2011;118(12):2368-2373.
20. Shih CY, Ritterband DC, Rubino S, et al. Visually significant and nonsignificant complications arising from Descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2009;148(6):837-843.